Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex
- PMID: 18188451
- PMCID: PMC2176193
- DOI: 10.1172/JCI33970
Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex
Abstract
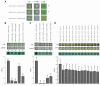
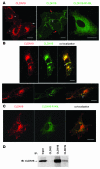
Tight junctions (TJs) play a key role in mediating paracellular ion reabsorption in the kidney. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) is an inherited disorder caused by mutations in the genes encoding the TJ proteins claudin-16 (CLDN16) and CLDN19; however, the mechanisms underlying the roles of these claudins in mediating paracellular ion reabsorption in the kidney are not understood. Here we showed that in pig kidney epithelial cells, CLDN19 functioned as a Cl(-) blocker, whereas CLDN16 functioned as a Na(+) channel. Mutant forms of CLDN19 that are associated with FHHNC were unable to block Cl(-) permeation. Coexpression of CLDN16 and CLDN19 generated cation selectivity of the TJ in a synergistic manner, and CLDN16 and CLDN19 were observed to interact using several criteria. In addition, disruption of this interaction by introduction of FHHNC-causing mutant forms of either CLDN16 or CLDN19 abolished their synergistic effect. Our data show that CLDN16 interacts with CLDN19 and that their association confers a TJ with cation selectivity, suggesting a mechanism for the role of mutant forms of CLDN16 and CLDN19 in the development of FHHNC.
Figures




References
-
- Simon D.B., et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. . Science. 1999;285:103–106. - PubMed
-
- Anderson J.M., Van Itallie C.M., Fanning A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 2004;16:140–145. - PubMed
-
- Hou J., Paul D.L., Goodenough D.A. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005;118:5109–5118. - PubMed
-
- Hou J., Gomes A.S., Paul D.L., Goodenough D.A. Study of claudin function by RNA interference. J. Biol. Chem. 2006;281:36117–36123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

