A computational study of the development of epithelial acini: I. Sufficient conditions for the formation of a hollow structure
- PMID: 18188652
- PMCID: PMC3812693
- DOI: 10.1007/s11538-007-9274-1
A computational study of the development of epithelial acini: I. Sufficient conditions for the formation of a hollow structure
Abstract
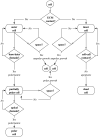
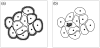
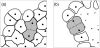
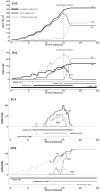
Normal hollow epithelial acini are 3-dimensional culture structures that resemble the architecture and functions of normal breast glands and lobules. This experimental model enables in vitro investigations of genotypic and molecular abnormalities associated with epithelial cancers. However, the way in which the acinar structure is formed is not yet completely understood. Gaining more information about consecutive stages of acini development-starting from a single cell that gives rise to a cluster of randomly oriented cells, followed by cell differentiation that leads to a layer of polarised cells enclosing the hollow lumen-will provide insight into the transformations of eukaryotic cells that are necessary for their successful arrangement into an epithelium. In this paper, we introduce a two-dimensional single-cell-based model representing the cross section of a typical acinus. Using this model, we investigate mechanisms that lead to the unpolarised cell growth, cell polarisation, stabilisation of the acinar structure and maintenance of the hollow lumen and discuss the sufficient conditions for each stage of acinar formation. In the follow-up paper (Rejniak and Anderson, A computational study of the development of epithelial acini. II. Necessary conditions for structure and lumen stability), we investigate what morphological changes are observable in the growing acini when some assumptions of this model are relaxed.
Figures










References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4th Garland Science; 2002.
-
- Anderson ARA. A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol. 2005;22:163–186. - PubMed
-
- Anderson ARA, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–915. - PubMed
-
- Ardens MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. - PubMed
-
- Bowen ID, Lockshin RA. Cell Death in Biology and Pathology. Kluwer Academic; Dordercht: 1981.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

