Defects in ciliary localization of Nek8 is associated with cystogenesis
- PMID: 18189147
- PMCID: PMC6890203
- DOI: 10.1007/s00467-007-0692-y
Defects in ciliary localization of Nek8 is associated with cystogenesis
Abstract
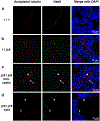
Mutations in the human NIMA (Never in Mitosis gene A)-related kinase 8 (Nek8) are associated with a rare form of the juvenile renal cystic disease, nephronophthisis type 9, and mutations in murine Nek8 cause renal cysts in jck mice. Cystogenesis involves dysfunctional ciliary signaling, and we have previously reported that Nek8 localizes to the primary cilium in mouse kidney epithelial cells. We now report that in developing mouse kidney, Nek8 is detected in the cilia of a subset of ureteric-bud-derived tubules at embryonic day (E)15.5. An increasing proportion of ureteric-bud-derived tubules express ciliary Nek8 until E18.5. Postnatal day 1 and 7 Nek8 is observed with equal frequency in both ureteric-bud and non-ureteric-bud-derived tubules. To investigate the cell biological consequences of kinase-deficient and jck mutant forms of Nek8, we transiently expressed green fluorescent protein (GFP)-tagged constructs in vitro. Mutations in the kinase and C-terminal domains of Nek8 adversely affected ciliary targeting but did not affect ciliogenesis or ciliary length. Consistent with these in vitro observations, kidneys from homozygous jck mice revealed reduced ciliary expression of Nek8 compared with kidneys from heterozygous (unaffected) mice. These data indicate that the ciliary localization of Nek8 in a subset of ureteric-bud-derived kidney tubules is essential for maintaining the integrity of those tubules in the mammalian kidney.
Figures





References
-
- Guay-Woodford LM (2006) Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol 21:1369–1376 - PubMed
-
- Hildebrandt F, Otto E (2005) Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6:928–940 - PubMed
-
- Pazour GJ (2004) Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol 15:2528–2536 - PubMed
-
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR (2002) A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in the zebrafish. Development 129:5839–5846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

