Species and density of implant surface chemistry affect the extent of foreign body reactions
- PMID: 18189430
- PMCID: PMC3230931
- DOI: 10.1021/la7025973
Species and density of implant surface chemistry affect the extent of foreign body reactions
Abstract
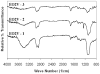
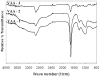
Implant-associated fibrotic capsule formation presents a major challenge for the development of long-term drug release microspheres and implantable sensors. Since material properties have been shown to affect in vitro cellular responses and also to influence short-term in vivo tissue responses, we have thus assumed that the type and density of surface chemical groups would affect the degree of tissue responses to microsphere implants. To test this hypothesis, polypropylene particles with different surface densities of -OH and -COOH groups, along with the polypropylene control (-CH2 groups) were utilized. The influence of functional groups and their surface densities on fibrotic reactions were analyzed using a mice subcutaneous implantation model. Our comparative studies included determination and correlation of the extents of fibrotic capsule formation, cell infiltration into the particles, and recruitment of CD11b+ inflammatory cells for all of the substrates employed. We have observed major differences among microspheres coated with different surface functionalities. Surfaces with -OH surface groups trigger the strongest responses, while -COOH-rich surfaces prompt the least tissue reactions. However, variation of the surface density of either functional group has a relatively minor influence on the extent of fibrotic tissue reactions. The present results show that surface functionality can be used as a powerful tool to alter implant-associated fibrotic reactions and, potentially, to improve the efficacy and function of drug-delivery microspheres, implantable sensors, and tissue-engineering scaffolds.
Figures
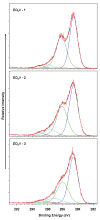
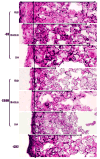
 ) shows the trend followed by capsule thickness and the extent of cell infiltration in to the implant across functional groups (Magnification 20x).
) shows the trend followed by capsule thickness and the extent of cell infiltration in to the implant across functional groups (Magnification 20x).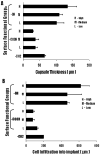

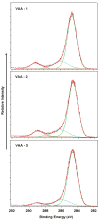
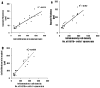
 ) shows the extent of collagen deposition. (Magnification 20x).
) shows the extent of collagen deposition. (Magnification 20x).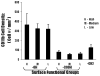
Similar articles
-
Surface chemistry influences implant-mediated host tissue responses.J Biomed Mater Res A. 2008 Sep;86(3):617-26. doi: 10.1002/jbm.a.31649. J Biomed Mater Res A. 2008. PMID: 18022841 Free PMC article.
-
An elastomer with in situ generated pure zwitterionic surfaces for fibrosis-resistant implants.Acta Biomater. 2024 Sep 1;185:226-239. doi: 10.1016/j.actbio.2024.06.047. Epub 2024 Jul 6. Acta Biomater. 2024. PMID: 38972625
-
Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model.Acta Biomater. 2014 May;10(5):1856-63. doi: 10.1016/j.actbio.2013.12.056. Epub 2014 Jan 7. Acta Biomater. 2014. PMID: 24406200
-
Surface chemistry influences implant biocompatibility.Curr Top Med Chem. 2008;8(4):270-80. doi: 10.2174/156802608783790901. Curr Top Med Chem. 2008. PMID: 18393890 Free PMC article. Review.
-
[Development of better tolerated prosthetic materials: applications in gynecological surgery].J Gynecol Obstet Biol Reprod (Paris). 2002 Oct;31(6):527-40. J Gynecol Obstet Biol Reprod (Paris). 2002. PMID: 12407323 Review. French.
Cited by
-
In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly(εCL)-b-Poly(EtOEP)-Based Films.Polymers (Basel). 2020 Dec 18;12(12):3039. doi: 10.3390/polym12123039. Polymers (Basel). 2020. PMID: 33353096 Free PMC article.
-
Host Response to Biomaterials for Cartilage Tissue Engineering: Key to Remodeling.Front Bioeng Biotechnol. 2021 May 4;9:664592. doi: 10.3389/fbioe.2021.664592. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34017827 Free PMC article. Review.
-
Insights in the host response towards biomaterial-based scaffolds for cancer therapy.Front Bioeng Biotechnol. 2023 Jun 5;11:1149943. doi: 10.3389/fbioe.2023.1149943. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37342507 Free PMC article. Review.
-
The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis.Biomaterials. 2012 Jan;33(3):876-85. doi: 10.1016/j.biomaterials.2011.10.002. Epub 2011 Oct 22. Biomaterials. 2012. PMID: 22019117 Free PMC article.
-
Biomaterials: Foreign Bodies or Tuners for the Immune Response?Int J Mol Sci. 2019 Feb 1;20(3):636. doi: 10.3390/ijms20030636. Int J Mol Sci. 2019. PMID: 30717232 Free PMC article. Review.
References
-
- Benagiano G, Gabelnick HL. J Steroid Biochem. 1979;11:449–455. - PubMed
-
- Benagiano G, Ermini M, Gabelnick HL. Hormonal factors in fertlity, infertility and contraception. In: Vander Molen HJ, Klopper A, Lunefeld BL, Neves e Castro M, Sciarro F, Vermeulen A, editors. Excerpta Medica: Amsterdam. 1982. p. 141.
-
- Ory SG, Hammond CB, Yancy SG, Hendern RW, Pitt CG. Am J Obstet Gynecol. 1983;145:600–605. - PubMed
-
- Qian F, Nasongkla N, Gao J. J Biomed Mater Res. 2002;61:203–211. - PubMed
-
- Qian F, Stowe N, Liu EH, Saidel GM, Gao J. J Cont Rel. 2003;91:157–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

