The CMV early enhancer/chicken beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors
- PMID: 18190688
- PMCID: PMC2254385
- DOI: 10.1186/1471-2121-9-2
The CMV early enhancer/chicken beta actin (CAG) promoter can be used to drive transgene expression during the differentiation of murine embryonic stem cells into vascular progenitors
Abstract
Background: Mouse embryonic stem cells cultured in vitro have the ability to differentiate into cells of the three germ layers as well as germ cells. The differentiation mimics early developmental events, including vasculogenesis and early angiogenesis and several differentiation systems are being used to identify factors that are important during the formation of the vascular system. Embryonic stem cells are difficult to transfect, while downregulation of promoter activity upon selection of stable transfectants has been reported, rendering the study of proteins by overexpression difficult.
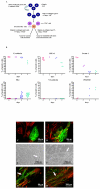
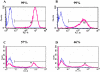
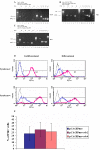
Results: CCE mouse embryonic stem cells were differentiated on collagen type IV for 4-5 days, Flk1+ mesodermal cells were sorted and replated either on collagen type IV in the presence of VEGFA to give rise to endothelial cells and smooth muscle cells or in collagen type I gels for the formation of vascular tubes. The activity of the CMV and beta-actin promoters was downregulated during selection of stable transfectants and during differentiation to the Flk1 stage, while the CMV immediate enhancer/beta-actin promoter in the pCAGIPuro-GFP vector led to 100% of stably transfected undifferentiated and differentiated cells expressing GFP. To further test this system we expressed syndecan-2 and -4 in these cells and demonstrated high levels of transgene expression in both undifferentiated cells and cells differentiated to the Flk1 stage.
Conclusion: Vectors containing the CAG promoter offer a valuable tool for the long term expression of transgenes during stem cell differentiation towards mesoderm, while the CMV and beta-actin promoters lead to very poor transgene expression during this process.
Figures


References
-
- Feraud O, Cao Y, Vittet D. Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest. 2001;81:1669–1681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

