Down-regulation of cell surface CXCR4 by HIV-1
- PMID: 18190699
- PMCID: PMC2248172
- DOI: 10.1186/1743-422X-5-6
Down-regulation of cell surface CXCR4 by HIV-1
Abstract
Background: CXC chemokine receptor 4 (CXCR4), a member of the G-protein-coupled chemokine receptor family, can serve as a co-receptor along with CD4 for entry into the cell of T-cell tropic X4 human immunodeficiency virus type 1 (HIV-1) strains. Productive infection of T-lymphoblastoid cells by X4 HIV-1 markedly reduces cell-surface expression of CD4, but whether or not the co-receptor CXCR4 is down-regulated has not been conclusively determined.
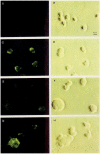
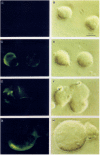
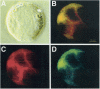
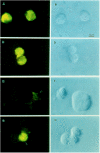
Results: Infection of human T-lymphoblastoid cell line RH9 with HIV-1 resulted in down-regulation of cell surface CXCR4 expression. Down-regulation of surface CXCR4 correlated temporally with the increase in HIV-1 protein expression. CXCR4 was concentrated in intracellular compartments in H9 cells after HIV-1 infection. Immunofluorescence microscopy studies showed that CXCR4 and HIV-1 glycoproteins were co-localized in HIV infected cells. Inducible expression of HIV-1 envelope glycoproteins also resulted in down-regulation of CXCR4 from the cell surface.
Conclusion: These results indicated that cell surface CXCR4 was reduced in HIV-1 infected cells, whereas expression of another membrane antigen, CD3, was unaffected. CXCR4 down-regulation may be due to intracellular sequestering of HIV glycoprotein/CXCR4 complexes.
Figures

References
-
- Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J, Rot A, Schall T, Tsang M, Thorpe R, Van Damme J, Wadhwa M, Yoshie O, Zlotnik A, Zoon K. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

