Point mutations in membrane proteins reshape energy landscape and populate different unfolding pathways
- PMID: 18191146
- PMCID: PMC2742699
- DOI: 10.1016/j.jmb.2007.12.027
Point mutations in membrane proteins reshape energy landscape and populate different unfolding pathways
Abstract
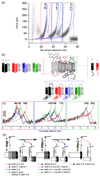
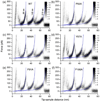
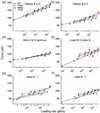
Using single-molecule force spectroscopy, we investigated the effect of single point mutations on the energy landscape and unfolding pathways of the transmembrane protein bacteriorhodopsin. We show that the unfolding energy barriers in the energy landscape of the membrane protein followed a simple two-state behavior and represent a manifestation of many converging unfolding pathways. Although the unfolding pathways of wild-type and mutant bacteriorhodopsin did not change, indicating the presence of same ensemble of structural unfolding intermediates, the free energies of the rate-limiting transition states of the bacteriorhodopsin mutants decreased as the distance of those transition states to the folded intermediate states decreased. Thus, all mutants exhibited Hammond behavior and a change in the free energies of the intermediates along the unfolding reaction coordinate and, consequently, their relative occupancies. This is the first experimental proof showing that point mutations can reshape the free energy landscape of a membrane protein and force single proteins to populate certain unfolding pathways over others.
Figures




References
-
- Daggett V, Fersht A. The present view of the mechanism of protein folding. Nat. Rev. Mol. Cell Biol. 2003;4:497–502. - PubMed
-
- Dill KA, Chan HS. From Levinthal to pathways to funnels. Nat. Struct. Biol. 1997;4:10–19. - PubMed
-
- Bowie JU. Solving the membrane protein folding problem. Nature. 2005;438:581–589. - PubMed
-
- White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 1999;28:319–365. - PubMed
-
- Daggett V, Fersht A. Is there a unifying mechanism for protein folding. Trends Biochem. Sci. 2003;28:19–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

