PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function
- PMID: 18191225
- PMCID: PMC2265257
- DOI: 10.1016/j.cell.2007.11.042
PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function
Abstract
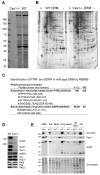
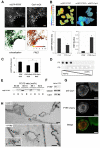
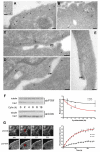
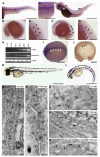
Caveolae are abundant cell-surface organelles involved in lipid regulation and endocytosis. We used comparative proteomics to identify PTRF (also called Cav-p60, Cavin) as a putative caveolar coat protein. PTRF-Cavin selectively associates with mature caveolae at the plasma membrane but not Golgi-localized caveolin. In prostate cancer PC3 cells, and during development of zebrafish notochord, lack of PTRF-Cavin expression correlates with lack of caveolae, and caveolin resides on flat plasma membrane. Expression of PTRF-Cavin in PC3 cells is sufficient to cause formation of caveolae. Knockdown of PTRF-Cavin reduces caveolae density, both in mammalian cells and in the zebrafish. Caveolin remains on the plasma membrane in PTRF-Cavin knockdown cells but exhibits increased lateral mobility and accelerated lysosomal degradation. We conclude that PTRF-Cavin is required for caveola formation and sequestration of mobile caveolin into immobile caveolae.
Figures



Comment in
-
PTRF triggers a cave in.Cell. 2008 Jan 11;132(1):23-4. doi: 10.1016/j.cell.2007.12.021. Cell. 2008. PMID: 18191216 Review.
References
-
- Clayton AH, Hanley QS, Verveer PJ. Graphical representation and multicomponent analysis of single-frequency fluorescence lifetime imaging microscopy data. J Microsc. 2004;213:1–5. - PubMed
-
- Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. - PubMed
-
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

