A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation
- PMID: 18191595
- PMCID: PMC2350190
- DOI: 10.1016/j.immuni.2007.11.020
A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation
Abstract
Engagement of the T cell antigen receptor (TCR) during antigen presentation initiates a coordinated action of a large number of signaling proteins and ion channels. AHNAK1 is a scaffold protein, highly expressed by CD4+ T cells, and is a critical component for calcium signaling. We showed that AHNAK1-deficient mice were highly susceptible to Leishmania major infection. AHNAK1-deficient CD4+ T cells responded poorly to TCR stimulation in vitro with low proliferation and low Interleukin-2 production. Furthermore, AHNAK1 deficiency resulted in a reduced calcium influx upon TCR crosslinking and subsequent poor activation of the transcription factor NFAT. AHNAK1 was required for plasma membrane expression of L-type calcium channels alpha 1S (Cav1.1), probably through its interaction with the beta regulatory subunit. Thus, AHNAK1 plays an essential role in T cell Ca2+ signaling through Cav1 channels, triggered via TCR activation; therefore, AHNAK1 is a potential target for therapeutic intervention.
Figures
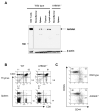
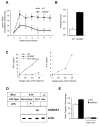

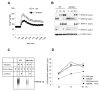

Comment in
-
Monster protein controls calcium entry and fights infection.Immunity. 2008 Jan;28(1):13-4. doi: 10.1016/j.immuni.2007.12.009. Immunity. 2008. PMID: 18199413
References
-
- Badou A, Matza D, Klemic KG, Govindaragulu U, Kaczmarek LK, Flavell RA. Requirement for Cav1.1 during TCR mediated calcium influx in T cells. 2007 manuscript submitted; enclosed for reviewers.
-
- Badou A, Savignac M, Moreau M, Leclerc C, Foucras G, Cassar G, Paulet P, Lagrange D, Druet P, Guery JC, Pelletier L. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur J Immunol. 2001;31:2487–2496. - PubMed
-
- Badou A, Savignac M, Moreau M, Leclerc C, Pasquier R, Druet P, Pelletier L. HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through L-type calcium channels. J Biol Chem. 1997;272:32411–32418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

