Staphylococcus aureus lipoproteins trigger human corneal epithelial innate response through toll-like receptor-2
- PMID: 18191935
- PMCID: PMC2408737
- DOI: 10.1016/j.micpath.2007.11.006
Staphylococcus aureus lipoproteins trigger human corneal epithelial innate response through toll-like receptor-2
Abstract
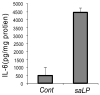
Bacterial lipoproteins (LP) are a family of cell wall components found in a wide variety of bacteria. In this study, we characterized the response of HUCL, a telomerase-immortalized human corneal epithelial cell (HCEC) line, to LP isolated from Staphylococcus (S) aureus. S. aureus LP (saLP) prepared by Triton X-114 extraction stimulated the activation of NF-kappaB, JNK, and P38 signaling pathways in HUCL cells. The extracts failed to stimulate NF-kappaB activation in HUCL cells after lipoprotein lipase treatment and in cell lines expressing TLR4 or TLR9, but not TLR2, indicating lipoprotein nature of the extracts. saLP induced the up-regulation of a variety of inflammatory cytokines and chemokines (IL-6, IL-8, ICAM-1), antimicrobial molecules (hBD-2, LL-37, and iNOS), and homeostasis genes (Mn-SOD) at both the mRNA level and protein level. Similar inflammatory response to saLP was also observed in primarily cultured HCECs using the production of IL-6 as readout. Moreover, TLR2 neutralizing antibody blocked the saLP-induced secretion of IL-6, IL-8 and hBD2 in HUCL cells. Our findings suggest that saLP activates TLR2 and triggers innate immune response in the cornea to S. aureus infection via production of proinflammatory cytokines and defense molecules.
Figures






References
-
- Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. - PubMed
-
- Jett BD, Gilmore MS. Host-parasite interactions in Staphylococcus aureus keratitis. DNA Cell Biol. 2002;21:397–404. - PubMed
-
- Cohen E, Laibson P, Arentsen J, Clemons C. Corneal ulcers associated with cosmetic extended wear soft contact lenses. Ophthalmology. 1987;94:109–114. - PubMed
-
- Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal integrity how the “window” stays clear. Prog Histochem Cytochem. 2001;36:185–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

