Nef enhances HIV-1 infectivity via association with the virus assembly complex
- PMID: 18191978
- PMCID: PMC2440657
- DOI: 10.1016/j.virol.2007.12.001
Nef enhances HIV-1 infectivity via association with the virus assembly complex
Abstract
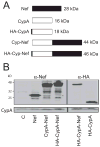
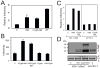
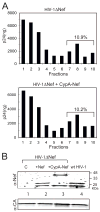
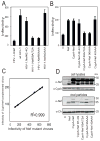
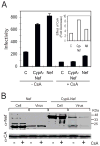
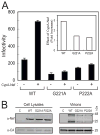
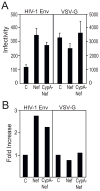
The HIV-1 accessory protein Nef enhances virus infectivity by facilitating an early post-entry step of infection. Nef acts in the virus producer cell, leading to a beneficial modification to HIV-1 particles. Nef itself is incorporated into HIV-1 particles, where it is cleaved by the viral protease during virion maturation. To probe the role of virion-associated Nef in HIV-1 infection, we generated a fusion protein consisting of the host protein cyclophilin A (CypA) linked to the amino terminus of Nef. The resulting CypA-Nef protein enhanced the infectivity of Nef-defective HIV-1 particles and was specifically incorporated into the virions via association with Gag during particle assembly. Pharmacologic or genetic inhibition of CypA-Nef binding to Gag prevented incorporation of CypA-Nef into virions and inhibited infectivity enhancement. Our results indicate that infectivity enhancement by Nef requires its association with a component of the assembling HIV-1 particle.
Figures
Similar articles
-
Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity.Virology. 1998 Aug 15;248(1):139-47. doi: 10.1006/viro.1998.9254. Virology. 1998. PMID: 9705263 Free PMC article.
-
Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity.J Virol. 1998 Nov;72(11):8833-40. doi: 10.1128/JVI.72.11.8833-8840.1998. J Virol. 1998. PMID: 9765428 Free PMC article.
-
The Antagonism of HIV-1 Nef to SERINC5 Particle Infectivity Restriction Involves the Counteraction of Virion-Associated Pools of the Restriction Factor.J Virol. 2016 Nov 14;90(23):10915-10927. doi: 10.1128/JVI.01246-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681140 Free PMC article.
-
Cell-dependent functional roles of HIV-1 Nef for virus replication (review).Int J Mol Med. 1999 Apr;3(4):427-30. doi: 10.3892/ijmm.3.4.427. Int J Mol Med. 1999. PMID: 10085418 Review.
-
The positive influence of Nef on viral infectivity.Res Virol. 1997 Jan-Feb;148(1):34-7. doi: 10.1016/s0923-2516(97)81910-3. Res Virol. 1997. PMID: 9017831 Review. No abstract available.
Cited by
-
Synthetic immunotherapy induces HIV virus specific Th1 cytotoxic response and death of an HIV-1 infected human cell line through classic complement activation.Virol J. 2013 Apr 4;10:107. doi: 10.1186/1743-422X-10-107. Virol J. 2013. PMID: 23557359 Free PMC article.
-
Implications of the nucleocapsid and the microenvironment in retroviral reverse transcription.Viruses. 2010 Apr;2(4):939-960. doi: 10.3390/v2040939. Epub 2010 Apr 2. Viruses. 2010. PMID: 21994662 Free PMC article.
-
Secretion modification region-derived peptide disrupts HIV-1 Nef's interaction with mortalin and blocks virus and Nef exosome release.J Virol. 2012 Jan;86(1):406-19. doi: 10.1128/JVI.05720-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013042 Free PMC article.
-
Interaction between Nef and INI1/SMARCB1 augments replicability of HIV-1 in resting human peripheral blood mononuclear cells.Arch Virol. 2015 Mar;160(3):727-37. doi: 10.1007/s00705-014-2315-9. Epub 2015 Jan 7. Arch Virol. 2015. PMID: 25559666 Free PMC article.
-
Cyclophilin A: a key player for human disease.Cell Death Dis. 2013 Oct 31;4(10):e888. doi: 10.1038/cddis.2013.410. Cell Death Dis. 2013. PMID: 24176846 Free PMC article. Review.
References
-
- Barnham KJ, Monks SA, Hinds MG, Azad AA, Norton RS. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36(20):5970–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

