Nef enhances HIV-1 infectivity via association with the virus assembly complex
- PMID: 18191978
- PMCID: PMC2440657
- DOI: 10.1016/j.virol.2007.12.001
Nef enhances HIV-1 infectivity via association with the virus assembly complex
Abstract
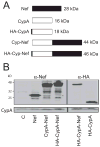
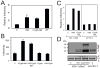
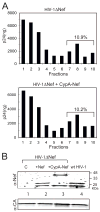
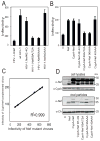
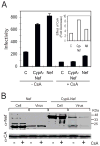
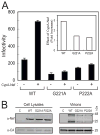
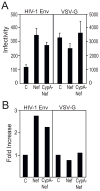
The HIV-1 accessory protein Nef enhances virus infectivity by facilitating an early post-entry step of infection. Nef acts in the virus producer cell, leading to a beneficial modification to HIV-1 particles. Nef itself is incorporated into HIV-1 particles, where it is cleaved by the viral protease during virion maturation. To probe the role of virion-associated Nef in HIV-1 infection, we generated a fusion protein consisting of the host protein cyclophilin A (CypA) linked to the amino terminus of Nef. The resulting CypA-Nef protein enhanced the infectivity of Nef-defective HIV-1 particles and was specifically incorporated into the virions via association with Gag during particle assembly. Pharmacologic or genetic inhibition of CypA-Nef binding to Gag prevented incorporation of CypA-Nef into virions and inhibited infectivity enhancement. Our results indicate that infectivity enhancement by Nef requires its association with a component of the assembling HIV-1 particle.
Figures
References
-
- Barnham KJ, Monks SA, Hinds MG, Azad AA, Norton RS. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36(20):5970–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

