Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS
- PMID: 18192221
- PMCID: PMC2722919
- DOI: 10.1152/ajpheart.00823.2007
Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS
Erratum in
- Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H576
Abstract
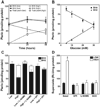
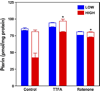
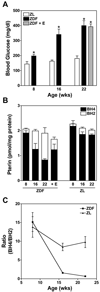
5,6,7,8-Tetrahydrobiopterin (BH(4)) is an essential cofactor of nitric oxide synthases (NOSs). Oxidation of BH(4), in the setting of diabetes and other chronic vasoinflammatory conditions, can cause cofactor insufficiency and uncoupling of endothelial NOS (eNOS), manifest by a switch from nitric oxide (NO) to superoxide production. Here we tested the hypothesis that eNOS uncoupling is not simply a consequence of BH(4) insufficiency, but rather results from a diminished ratio of BH(4) vs. its catalytically incompetent oxidation product, 7,8-dihydrobiopterin (BH(2)). In support of this hypothesis, [(3)H]BH(4) binding studies revealed that BH(4) and BH(2) bind eNOS with equal affinity (K(d) approximately 80 nM) and BH(2) can rapidly and efficiently replace BH(4) in preformed eNOS-BH(4) complexes. Whereas the total biopterin pool of murine endothelial cells (ECs) was unaffected by 48-h exposure to diabetic glucose levels (30 mM), BH(2) levels increased from undetectable to 40% of total biopterin. This BH(2) accumulation was associated with diminished calcium ionophore-evoked NO activity and accelerated superoxide production. Since superoxide production was suppressed by NOS inhibitor treatment, eNOS was implicated as a principal superoxide source. Importantly, BH(4) supplementation of ECs (in low and high glucose-containing media) revealed that calcium ionophore-evoked NO bioactivity correlates with intracellular BH(4):BH(2) and not absolute intracellular levels of BH(4). Reciprocally, superoxide production was found to negatively correlate with intracellular BH(4):BH(2). Hyperglycemia-associated BH(4) oxidation and NO insufficiency was recapitulated in vivo, in the Zucker diabetic fatty rat model of type 2 diabetes. Together, these findings implicate diminished intracellular BH(4):BH(2), rather than BH(4) depletion per se, as the molecular trigger for NO insufficiency in diabetes.
Figures




References
-
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. - PubMed
-
- Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, Pillai R, Channon KM. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116:2851–2859. - PubMed
-
- Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. - PubMed
-
- Britten MB, Zeiher AM, Schachinger V. Effects of cardiovascular risk factors on coronary artery remodeling in patients with mild atherosclerosis. Coron Artery Dis. 2003;14:415–422. - PubMed
-
- Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94:377–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

