Mutational analysis of Deinococcus radiodurans bacteriophytochrome reveals key amino acids necessary for the photochromicity and proton exchange cycle of phytochromes
- PMID: 18192276
- PMCID: PMC2431007
- DOI: 10.1074/jbc.M709355200
Mutational analysis of Deinococcus radiodurans bacteriophytochrome reveals key amino acids necessary for the photochromicity and proton exchange cycle of phytochromes
Abstract
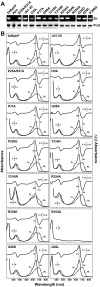
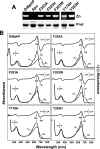
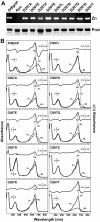
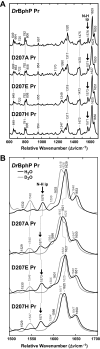
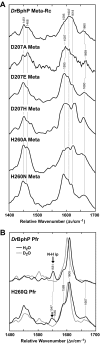
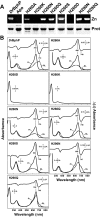
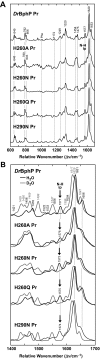
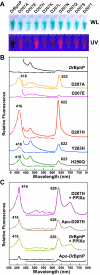
The ability of phytochromes (Phy) to act as photointerconvertible light switches in plants and microorganisms depends on key interactions between the bilin chromophore and the apoprotein that promote bilin attachment and photointerconversion between the spectrally distinct red light-absorbing Pr conformer and far red light-absorbing Pfr conformer. Using structurally guided site-directed mutagenesis combined with several spectroscopic methods, we examined the roles of conserved amino acids within the bilin-binding domain of Deinococcus radiodurans bacteriophytochrome with respect to chromophore ligation and Pr/Pfr photoconversion. Incorporation of biliverdin IXalpha (BV), its structure in the Pr state, and its ability to photoisomerize to the first photocycle intermediate are insensitive to most single mutations, implying that these properties are robust with respect to small structural/electrostatic alterations in the binding pocket. In contrast, photoconversion to Pfr is highly sensitive to the chromophore environment. Many of the variants form spectrally bleached Meta-type intermediates in red light that do not relax to Pfr. Particularly important are Asp-207 and His-260, which are invariant within the Phy superfamily and participate in a unique hydrogen bond matrix involving the A, B, and C pyrrole ring nitrogens of BV and their associated pyrrole water. Resonance Raman spectroscopy demonstrates that substitutions of these residues disrupt the Pr to Pfr protonation cycle of BV with the chromophore locked in a deprotonated Meta-R(c)-like photoconversion intermediate after red light irradiation. Collectively, the data show that a number of contacts contribute to the unique photochromicity of Phy-type photoreceptors. These include residues that fix the bilin in the pocket, coordinate the pyrrole water, and possibly promote the proton exchange cycle during photoconversion.
Figures
References
-
- Vierstra, R. D., and Karniol, B. (2005) in Handbook of Photosensory Receptors (Briggs, W. R., and Spudich, J. L., eds) pp. 171–196, Wiley-VCH Press, Weinheim, Germany
-
- Quail, P. H. (2002) Nat. Rev. Mol. Cell Biol. 3 85–93 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

