Alpha2delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes
- PMID: 18192372
- PMCID: PMC2275671
- DOI: 10.1529/biophysj.107.118893
Alpha2delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes
Abstract
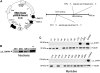
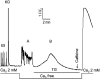
It has been shown that small interfering RNA (siRNA) partial knockdown of the alpha(2)delta(1) dihydropyridine receptor subunits cause a significant increase in the rate of activation of the L-type Ca(2+) current in myotubes but have little or no effect on skeletal excitation-contraction coupling. This study used permanent siRNA knockdown of alpha(2)delta(1) to address two important unaddressed questions. First, does the alpha(2)delta(1) subunit contribute to the size and/or spacing of tetradic particles? Second, is the alpha(2)delta(1) subunit important for excitation-coupled calcium entry? We found that the size and spacing of tetradic particles is unaffected by siRNA knockdown of alpha(2)delta(1), indicating that the visible particle represents the alpha(1s) subunit. Strikingly, >97% knockdown of alpha(2)delta(1) leads to a complete loss of excitation-coupled calcium entry during KCl depolarization and a more rapid decay of Ca(2+) transients during bouts of repetitive electrical stimulation like those occurring during normal muscle activation in vivo. Thus, we conclude that the alpha(2)delta(1) dihydropyridine receptor subunit is physiologically necessary for sustaining Ca(2+) transients in response to prolonged depolarization or repeated trains of action potentials.
Figures

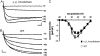



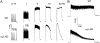

References
-
- Nakai, J., R. T. Dirksen, H. T. Nguyen, I. N. Pessah, K. G. Beam, and P. D. Allen. 1996. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 380:72–75. - PubMed
-
- Grabner, M., R. T. Dirksen, N. Suda, and K. G. Beam. 1999. The II–III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 274:21913–21919. - PubMed
-
- Hurne, A. M., J. J. O'Brien, D. Wingrove, G. Cherednichenko, P. D. Allen, K. G. Beam, and I. N. Pessah. 2005. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J. Biol. Chem. 280:36994–37004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

