The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance
- PMID: 18192383
- PMCID: PMC2258881
- DOI: 10.1128/JB.01740-07
The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance
Abstract
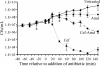
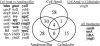
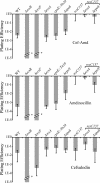
Gram-negative bacteria possess stress responses to maintain the integrity of the cell envelope. Stress sensors monitor outer membrane permeability, envelope protein folding, and energization of the inner membrane. The systems used by gram-negative bacteria to sense and combat stress resulting from disruption of the peptidoglycan layer are not well characterized. The peptidoglycan layer is a single molecule that completely surrounds the cell and ensures its structural integrity. During cell growth, new peptidoglycan subunits are incorporated into the peptidoglycan layer by a series of enzymes called the penicillin-binding proteins (PBPs). To explore how gram-negative bacteria respond to peptidoglycan stress, global gene expression analysis was used to identify Escherichia coli stress responses activated following inhibition of specific PBPs by the beta-lactam antibiotics amdinocillin (mecillinam) and cefsulodin. Inhibition of PBPs with different roles in peptidoglycan synthesis has different consequences for cell morphology and viability, suggesting that not all perturbations to the peptidoglycan layer generate equivalent stresses. We demonstrate that inhibition of different PBPs resulted in both shared and unique stress responses. The regulation of capsular synthesis (Rcs) phosphorelay was activated by inhibition of all PBPs tested. Furthermore, we show that activation of the Rcs phosphorelay increased survival in the presence of these antibiotics, independently of capsule synthesis. Both activation of the phosphorelay and survival required signal transduction via the outer membrane lipoprotein RcsF and the response regulator RcsB. We propose that the Rcs pathway responds to peptidoglycan damage and contributes to the intrinsic resistance of E. coli to beta-lactam antibiotics.
Figures

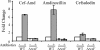

References
-
- Bolstad, B. M., R. A. Irizarrt, M. Astrand, and T. P. Speed. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19185-193. - PubMed
-
- Boulanger, A., A. Francez-Charlot, A. Conter, M.-P. Castanie-Cornet, K. Cam, and C. Gutierrez. 2005. Multistress regulation in Escherichia coli: expression of osmB involves two independent promoters responding either to σS or to the RcsCDB His-Asp phosphorelay. J. Bacteriol. 1873282-3286. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

