Protein kinase Cdelta regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation
- PMID: 18192589
- PMCID: PMC3970932
- DOI: 10.1152/ajplung.00353.2007
Protein kinase Cdelta regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation
Retraction in
-
Retraction. Protein kinase Cδ regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation.Am J Physiol Lung Cell Mol Physiol. 2011 Dec;301(6):L1006. doi: 10.1152/ajplung.zh5-6007-corr.2011. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 22142987 Free PMC article. No abstract available.
Abstract
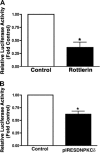
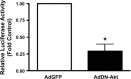
In this study, we explore the roles of the delta isoform of PKC (PKCdelta) in the regulation of endothelial nitric oxide synthase (eNOS) activity in pulmonary arterial endothelial cells isolated from fetal lambs (FPAECs). Pharmacological inhibition of PKCdelta with either rottlerin or with the peptide, deltaV1-1, acutely attenuated NO production, and this was associated with a decrease in phosphorylation of eNOS at Ser1177 (S1177). The chronic effects of PKCdelta inhibition using either rottlerin or the overexpression of a dominant negative PKCdelta mutant included the downregulation of eNOS gene expression that was manifested by a decrease in both eNOS promoter activity and protein expression after 24 h of treatment. We also found that PKCdelta inhibition blunted Akt activation as observed by a reduction in phosphorylated Akt at position Ser473. Thus, we conclude that PKCdelta is actively involved in the activation of Akt. To determine the effect of Akt on eNOS signaling, we overexpressed a dominant negative mutant of Akt and determined its effect of NO generation, eNOS expression, and phosphorylation of eNOS at S1177. Our results demonstrated that Akt inhibition was associated with decreased NO production that correlated with reduced phosphorylation of eNOS at S1177, and decreased eNOS promoter activity. We next evaluated the effect of endogenously produced NO on eNOS expression by incubating FPAECs with the eNOS inhibitor 2-ethyl-2-thiopseudourea (ETU). ETU significantly inhibited NO production, eNOS promoter activity, and eNOS protein levels. Together, our data indicate involvement of PKCdelta-mediated Akt activation and NO generation in maintaining eNOS expression.
Figures







References
-
- Ahn BK, Jeong SK, Kim HS, Choi KJ, Seo JT, Choi EH, Ahn SK, Lee SH. Rottlerin, a specific inhibitor of protein kinase C-delta, impedes barrier repair response by increasing intracellular free calcium. J Invest Dermatol 126: 1348–1355, 2006 - PubMed
-
- Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003 - PubMed
-
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

