Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival
- PMID: 18193038
- PMCID: PMC3153364
- DOI: 10.1038/ncb1677
Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival
Erratum in
- Nat Cell Biol. 2008 Mar;10(3):370
Abstract
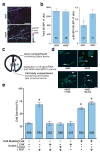
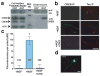
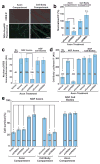
During development of the nervous system, axons and growth cones contain mRNAs such as beta-actin, cofilin and RhoA, which are locally translated in response to guidance cues. Intra-axonal translation of these mRNAs results in local morphological responses; however, other functions of intra-axonal mRNA translation remain unknown. Here, we show that axons of developing mammalian neurons contain mRNA encoding the cAMP-responsive element (CRE)-binding protein (CREB). CREB is translated within axons in response to nerve growth factor (NGF) and is retrogradely trafficked to the cell body. In neurons that are selectively deficient in axonal CREB transcripts, increases in nuclear pCREB, CRE-mediated transcription and neuronal survival elicited by axonal application of NGF are abolished, indicating a signalling function for axonally synthesized CREB. These studies identify a signalling role for axonally derived CREB, and indicate that signal-dependent synthesis and retrograde trafficking of transcription factors enables specific transcriptional responses to signalling events at distal axons.
Figures


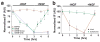


Comment in
-
Outsourcing CREB translation to axons to survive.Nat Cell Biol. 2008 Feb;10(2):115-8. doi: 10.1038/ncb0208-115. Nat Cell Biol. 2008. PMID: 18246035 Free PMC article.
References
-
- Czaplinski K, Singer RH. Pathways for mRNA localization in the cytoplasm. Trends Biochem Sci. 2006;31:687–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

