G-protein-coupled receptor phosphorylation: where, when and by whom
- PMID: 18193069
- PMCID: PMC2268057
- DOI: 10.1038/sj.bjp.0707662
G-protein-coupled receptor phosphorylation: where, when and by whom
Abstract
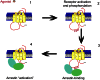
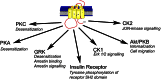
Almost all G-protein coupled receptors (GPCRs) are regulated by phosphorylation and this process is a key event in determining the signalling properties of this receptor super-family. Receptors are multiply phosphorylated at sites that can occur throughout the intracellular regions of the receptor. This diversity of phospho-acceptor sites together with a lack of consensus phosphorylation sequences has led to the suggestion that the precise site of phosphorylation is not important in the phosphorylation-dependent regulation of GPCR function but rather it is the increase in bulk negative charge of the intracellular face of the receptor which is the significant factor. This review investigates the possibility that the multi-site nature of GPCR phosphorylation reflects the importance of specific phosphorylation events which mediate distinct signalling outcomes. In this way receptor phosphorylation may provide for a flexible regulatory mechanism that can be tailored in a tissue specific manner to regulate physiological processes. By understanding the flexible nature of GPCR phosphorylation if may be possible to develop agonists or allosteric modulators that promote a subset of phosphorylation events on the target GPCR and thereby restrict the action of the drug to a particular receptor mediated signalling response.
Figures

References
-
- Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. - PMC - PubMed
-
- Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, et al. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985;260:7094–7101. - PubMed
-
- Blaukat A, Pizard A, Breit A, Wernstedt C, Alhenc-Gelas F, Muller-Esterl W, et al. Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function. J Biol Chem. 2001;276:40431–40440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

