Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen
- PMID: 18193943
- PMCID: PMC2186359
- DOI: 10.1371/journal.ppat.0040006
Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen
Abstract
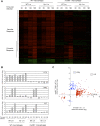
How the innate immune system tailors specific responses to diverse microbial infections is not well understood. Cells use a limited number of host receptors and signaling pathways to both discriminate among extracellular and intracellular microbes, and also to generate responses commensurate to each threat. Here, we have addressed these questions by using DNA microarrays to monitor the macrophage transcriptional response to the intracellular bacterial pathogen Listeria monocytogenes. By utilizing combinations of host and bacterial mutants, we have defined the host transcriptional responses to vacuolar and cytosolic bacteria. These compartment-specific host responses induced significantly different sets of target genes, despite activating similar transcription factors. Vacuolar signaling was entirely MyD88-dependent, and induced the transcription of pro-inflammatory cytokines. The IRF3-dependent cytosolic response induced a distinct set of target genes, including IFNbeta. Many of these cytosolic response genes were induced by secreted cytokines, so we further identified those host genes induced independent of secondary signaling. The host response to cytosolic bacteria was reconstituted by the cytosolic delivery of L. monocytogenes genomic DNA, but we observed an amplification of this response by NOD2 signaling in response to MDP. Correspondingly, the induction of IFNbeta was reduced in nod2-/- macrophages during infection with either L. monocytogenes or Mycobacterium tuberculosis. Combinatorial control of IFNbeta induction by recognition of both DNA and MDP may highlight a mechanism by which the innate immune system integrates the responses to multiple ligands presented in the cytosol by intracellular pathogens.
Conflict of interest statement
Figures


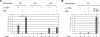



References
-
- Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187(Suppl 2):S340–S345. - PubMed
-
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. - PubMed
-
- Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. - DOI - PMC - PubMed
-
- Medzhitov R, Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. - PubMed
-
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

