The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment
- PMID: 18193945
- PMCID: PMC2174975
- DOI: 10.1371/journal.pgen.0040006
The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment
Abstract
Mutations in optic atrophy 1 (OPA1), a nuclear gene encoding a mitochondrial protein, is the most common cause for autosomal dominant optic atrophy (DOA). The condition is characterized by gradual loss of vision, color vision defects, and temporal optic pallor. To understand the molecular mechanism by which OPA1 mutations cause optic atrophy and to facilitate the development of an effective therapeutic agent for optic atrophies, we analyzed phenotypes in the developing and adult Drosophila eyes produced by mutant dOpa1 (CG8479), a Drosophila ortholog of human OPA1. Heterozygous mutation of dOpa1 by a P-element or transposon insertions causes no discernable eye phenotype, whereas the homozygous mutation results in embryonic lethality. Using powerful Drosophila genetic techniques, we created eye-specific somatic clones. The somatic homozygous mutation of dOpa1 in the eyes caused rough (mispatterning) and glossy (decreased lens and pigment deposition) eye phenotypes in adult flies; this phenotype was reversible by precise excision of the inserted P-element. Furthermore, we show the rough eye phenotype is caused by the loss of hexagonal lattice cells in developing eyes, suggesting an increase in lattice cell apoptosis. In adult flies, the dOpa1 mutation caused an increase in reactive oxygen species (ROS) production as well as mitochondrial fragmentation associated with loss and damage of the cone and pigment cells. We show that superoxide dismutase 1 (SOD1), Vitamin E, and genetically overexpressed human SOD1 (hSOD1) is able to reverse the glossy eye phenotype of dOPA1 mutant large clones, further suggesting that ROS play an important role in cone and pigment cell death. Our results show dOpa1 mutations cause cell loss by two distinct pathogenic pathways. This study provides novel insights into the pathogenesis of optic atrophy and demonstrates the promise of antioxidants as therapeutic agents for this condition.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures






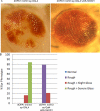
References
-
- Bette S, Schlaszus H, Wissinger B, Meyermann R, Mittelbronn M. OPA1, associated with autosomal dominant optic atrophy, is widely expressed in the human brain. Acta Neuropathol (Berl) 2005;109:393–399. - PubMed
-
- Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. II. Clinical and epidemiological aspects. Acta Ophthalmol Scand. 1996;74:3–7. - PubMed
-
- Votruba M, Aijaz S, Moore AT. A review of primary hereditary optic neuropathies. J Inherit Metab Dis. 2003;26:209–227. - PubMed
-
- Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

