Migration of Apicomplexa across biological barriers: the Toxoplasma and Plasmodium rides
- PMID: 18194412
- PMCID: PMC2329915
- DOI: 10.1111/j.1600-0854.2008.00703.x
Migration of Apicomplexa across biological barriers: the Toxoplasma and Plasmodium rides
Abstract
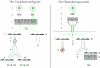
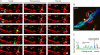
The invasive stages of Apicomplexa parasites, called zoites, have been largely studied in in vitro systems, with a special emphasis on their unique gliding and host cell invasive capacities. In contrast, the means by which these parasites reach their destination in their hosts are still poorly understood. We summarize here our current understanding of the cellular basis of in vivo parasitism by two well-studied Apicomplexa zoites, the Toxoplasma tachyzoite and the Plasmodium sporozoite. Despite being close relatives, these two zoites use different strategies to reach their goal and establish infection.
Figures


References
-
- Dubremetz JF, Garcia-Réguet N, Conseil V, Fourmaux MN. Apical organelles and host cell invasion by Apicomplexa. Int J Parasitol. 1998;28:1007–1013. - PubMed
-
- Heintzelman MB. Cellular and molecular mechanisms of gliding locomotion in eukaryotes. Int Rev Cytol. 2006;251:79–129. - PubMed
-
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. - PubMed
-
- Fowler RE, Margos G, Mitchell GH. The cytoskeleton and motility in apicomplexan invasion. Adv Parasitol. 2004;56:213–263. - PubMed
-
- Mota MM, Rodriguez A. Migration through host cells by apicomplexan parasites. Microbes Infect. 2001;3:1123–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

