The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo
- PMID: 18194466
- PMCID: PMC3828891
- DOI: 10.1111/j.1582-4934.2008.00215.x
The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo
Abstract
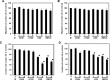
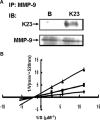
RECK is a membrane-anchored glycoprotein which may negatively regulate matrix metalloproteinase (MMP) activity to suppress tumor invasion and metastasis. In this study, recombinant proteins corresponding to the residues 285-368 (named as CKM which contained cysteine knot motif), 605-799 (named as K123 which contained three Kazal motifs), 676-799 (named as K23 which contained the last two Kazal motifs) and full-length RECK were produced and their anti-cancer effects were tested. Full-length RECK and K23 but not K123 and CKM inhibited MMP9 secretion and activity. In addition, RECK and K23 inhibited invasion but not migration of metastatic lung cancer cells in vitro. Protein binding and kinetic study indicated that K23 physically interacted with MMP-9 and inhibited its activity by a non-competitive manner. Moreover, K23 reduced metastatic tumor growth in lungs of nude mice. Taken together, our results suggest that the K23 motifs of RECK protein can inhibit MMP-9 secretion and activity and attenuate metastasis of lung cancer cells.
Figures



References
-
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. - PubMed
-
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–85. - PubMed
-
- Visse R, Nagase H. Matrix metallopro-teinases and tissue inhibitors of metallo-proteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

