Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes
- PMID: 18194989
- PMCID: PMC3031334
- DOI: 10.1093/cvr/cvn008
Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes
Abstract
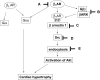
Aims: Cardiac hypertrophy by activation of the beta-adrenergic receptor (beta AR) is mediated more efficiently by the beta1-AR than by the beta2-AR. We investigated the signalling mechanism by which the beta1-AR mediates cardiac hypertrophy.
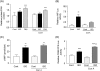
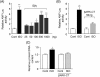
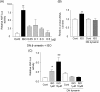
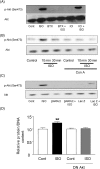
Methods and results: Experiments were performed in cultured neonatal rat cardiomyocytes. Hypertrophy was determined by the protein/DNA content and atrial natriuretic factor transcription. Phosphorylation of Akt and Src was assessed by immunoblotting. Isoproterenol (ISO, 10 microM), a non-selective beta-AR agonist, caused selective downregulation of the beta1-AR (control beta1 vs. beta2: 35 vs. 65%, Bmax 78 +/- 4 fmol/mg; 4 h, 10 vs. 90%, 61 +/- 5 fmol/mg). Concanavalin A (Con A, 0.5 microg/mL), an inhibitor of endocytosis, prevented downregulation of beta1-ARs by ISO treatment (4 h, 35 vs. 65%, 73 +/- 8 fmol/mg), suggesting that beta1-ARs selectively undergo endocytosis. Interference with beta1-AR endocytosis by Con A, carboxyl terminal peptide of beta-AR kinase-1, dominant negative (DN) beta-arrestin-1, or DN dynamin inhibited beta-adrenergic hypertrophy, suggesting that the endocytosis machinery plays a key role in mediating beta-adrenergic hypertrophy. Activation of Akt by the beta1-AR was blocked by inhibition of the endocytosis machinery, suggesting that endocytosis mediates activation of Akt. Akt plays a critical role in beta-adrenergic hypertrophy, since DN Akt blocked ISO-induced hypertrophy. beta-Adrenergic activation of Akt is mediated by Src, which associates with the endocytosis machinery and is necessary and sufficient to mediate beta-adrenergic hypertrophy.
Conclusion: Activation of the endocytosis machinery is required for activation of Akt, which, in turn, critically mediates beta1-AR-induced cardiac hypertrophy.
Figures

Comment in
-
Novel signalling cascade for cardiac hypertrophy activation by uncoupling and internalization of beta1-adrenoceptors.Cardiovasc Res. 2008 Apr 1;78(1):5-7. doi: 10.1093/cvr/cvn042. Epub 2008 Feb 15. Cardiovasc Res. 2008. PMID: 18281371 No abstract available.
Similar articles
-
Novel signalling cascade for cardiac hypertrophy activation by uncoupling and internalization of beta1-adrenoceptors.Cardiovasc Res. 2008 Apr 1;78(1):5-7. doi: 10.1093/cvr/cvn042. Epub 2008 Feb 15. Cardiovasc Res. 2008. PMID: 18281371 No abstract available.
-
Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart.J Mol Cell Cardiol. 2001 Mar;33(3):561-73. doi: 10.1006/jmcc.2000.1332. J Mol Cell Cardiol. 2001. PMID: 11181023
-
Phosphorylation of eukaryotic translation initiation factor 2Bepsilon by glycogen synthase kinase-3beta regulates beta-adrenergic cardiac myocyte hypertrophy.Circ Res. 2004 Apr 16;94(7):926-35. doi: 10.1161/01.RES.0000124977.59827.80. Epub 2004 Mar 4. Circ Res. 2004. PMID: 15001529
-
Modification of beta-adrenoceptor signal transduction pathway by genetic manipulation and heart failure.Mol Cell Biochem. 2000 Nov;214(1-2):131-55. doi: 10.1023/a:1007131925048. Mol Cell Biochem. 2000. PMID: 11195784 Review.
-
Therapeutic synergy and complementarity for ischemia/reperfusion injury: β1-adrenergic blockade and phosphodiesterase-3 inhibition.Int J Cardiol. 2016 Jul 1;214:374-80. doi: 10.1016/j.ijcard.2016.03.200. Epub 2016 Apr 3. Int J Cardiol. 2016. PMID: 27085132 Review.
Cited by
-
Cross-regulation between beta 1- and beta 3-adrenoceptors following chronic beta-adrenergic stimulation in neonatal rat cardiomyocytes.Br J Pharmacol. 2009 Sep;158(1):300-13. doi: 10.1111/j.1476-5381.2009.00328.x. Br J Pharmacol. 2009. PMID: 19719783 Free PMC article.
-
Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway.Int J Biol Sci. 2011 Apr 7;7(3):383-9. doi: 10.7150/ijbs.7.383. Int J Biol Sci. 2011. PMID: 21494433 Free PMC article.
-
Insulin and β Adrenergic Receptor Signaling: Crosstalk in Heart.Trends Endocrinol Metab. 2017 Jun;28(6):416-427. doi: 10.1016/j.tem.2017.02.002. Epub 2017 Feb 28. Trends Endocrinol Metab. 2017. PMID: 28256297 Free PMC article. Review.
-
Adrenoceptor Desensitization: Current Understanding of Mechanisms.Pharmacol Rev. 2024 May 2;76(3):358-387. doi: 10.1124/pharmrev.123.000831. Pharmacol Rev. 2024. PMID: 38697858 Free PMC article. Review.
-
Biased activation of β2-AR/Gi/GRK2 signal pathway attenuated β1-AR sustained activation induced by β1-adrenergic receptor autoantibody.Cell Death Discov. 2021 Nov 8;7(1):340. doi: 10.1038/s41420-021-00735-2. Cell Death Discov. 2021. PMID: 34750352 Free PMC article.
References
-
- Vatner SF, Vatner DE, Homcy CJ. Beta-adrenergic receptor signaling: an acute compensatory adjustment—inappropriate for the chronic stress of heart failure? Insights from Gsalpha overexpression and other genetically engineered animal models. Circ Res. 2000;86:502–506. - PubMed
-
- Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905. - PubMed
-
- Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

