Burkholderia cenocepacia requires the RpoN sigma factor for biofilm formation and intracellular trafficking within macrophages
- PMID: 18195023
- PMCID: PMC2258854
- DOI: 10.1128/IAI.01167-07
Burkholderia cenocepacia requires the RpoN sigma factor for biofilm formation and intracellular trafficking within macrophages
Abstract
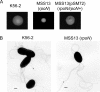
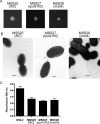
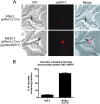
Chronic respiratory infections by Burkholderia cenocepacia in cystic fibrosis patients are associated with increased morbidity and mortality, but virulence factors determining the persistence of the infection in the airways are not well characterized. Using a chronic pulmonary infection model, we previously identified an attenuated mutant with an insertion in a gene encoding an RpoN activator protein, suggesting that RpoN and/or components of the RpoN regulon play a role in B. cenocepacia virulence. In this study, we demonstrate that a functional rpoN gene is required for bacterial motility and biofilm formation in B. cenocepacia K56-2. Unlike other bacteria, RpoN does not control flagellar biosynthesis, as evidenced by the presence of flagella in the rpoN mutant. We also demonstrate that, in macrophages, the rpoN mutant is rapidly trafficked to lysosomes while intracellular wild-type B. cenocepacia localizes in bacterium-containing vacuoles that exhibit a pronounced delay in phagolysosomal fusion. Rapid trafficking to the lysosomes is also associated with the release of red fluorescent protein into the vacuolar lumen, indicating loss of bacterial cell envelope integrity. Although a role for RpoN in motility and biofilm formation has been previously established, this study is the first demonstration that the RpoN regulon in B. cenocepacia is involved in delaying phagolysosomal fusion, thereby prolonging bacterial intracellular survival within macrophages.
Figures



Similar articles
-
Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles.Cell Microbiol. 2007 Jan;9(1):40-53. doi: 10.1111/j.1462-5822.2006.00766.x. Epub 2006 Jul 26. Cell Microbiol. 2007. PMID: 16869828
-
A type IV secretion system contributes to intracellular survival and replication of Burkholderia cenocepacia.Infect Immun. 2008 Dec;76(12):5447-55. doi: 10.1128/IAI.00451-08. Epub 2008 Sep 29. Infect Immun. 2008. PMID: 18824538 Free PMC article.
-
The Burkholderia cenocepacia K56-2 pleiotropic regulator Pbr, is required for stress resistance and virulence.Microb Pathog. 2010 May;48(5):168-77. doi: 10.1016/j.micpath.2010.02.006. Epub 2010 Mar 3. Microb Pathog. 2010. PMID: 20206249
-
The multifarious, multireplicon Burkholderia cepacia complex.Nat Rev Microbiol. 2005 Feb;3(2):144-56. doi: 10.1038/nrmicro1085. Nat Rev Microbiol. 2005. PMID: 15643431 Review.
-
Social interactions in the Burkholderia cepacia complex: biofilms and quorum sensing.Future Microbiol. 2010 Jul;5(7):1087-99. doi: 10.2217/fmb.10.68. Future Microbiol. 2010. PMID: 20632807 Review.
Cited by
-
Burkholderia cepacia Complex: Emerging Multihost Pathogens Equipped with a Wide Range of Virulence Factors and Determinants.Int J Microbiol. 2011;2011:607575. doi: 10.1155/2011/607575. Epub 2010 Aug 3. Int J Microbiol. 2011. PMID: 20811541 Free PMC article.
-
σ54-Dependent Response to Nitrogen Limitation and Virulence in Burkholderia cenocepacia Strain H111.Appl Environ Microbiol. 2015 Jun 15;81(12):4077-89. doi: 10.1128/AEM.00694-15. Epub 2015 Apr 3. Appl Environ Microbiol. 2015. PMID: 25841012 Free PMC article.
-
Burkholderia cenocepacia Lipopolysaccharide Modification and Flagellin Glycosylation Affect Virulence but Not Innate Immune Recognition in Plants.mBio. 2015 Jun 4;6(3):e00679. doi: 10.1128/mBio.00679-15. mBio. 2015. PMID: 26045541 Free PMC article.
-
Burkholderia cepacia Complex Vaccines: Where Do We Go from here?Vaccines (Basel). 2016 Apr 15;4(2):10. doi: 10.3390/vaccines4020010. Vaccines (Basel). 2016. PMID: 27092530 Free PMC article. Review.
-
An Oxygen-Sensing Two-Component System in the Burkholderia cepacia Complex Regulates Biofilm, Intracellular Invasion, and Pathogenicity.PLoS Pathog. 2017 Jan 3;13(1):e1006116. doi: 10.1371/journal.ppat.1006116. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28046077 Free PMC article.
References
-
- Al-Younes, H. M., T. Rudel, and T. F. Meyer. 1999. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell. Microbiol. 1237-247. - PubMed
-
- Berg, G., L. Eberl, and A. Hartmann. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 71673-1685. - PubMed
-
- Bittner, M., S. Saldías, C. Estevez, M. Zaldivar, C. L. Marolda, M. A. Valvano, and I. Contreras. 2002. O-antigen expression in Salmonella enterica serovar Typhi is regulated by nitrogen availability through RpoN-mediated transcriptional control of the rfaH gene. Microbiology 1483789-3799. - PubMed
-
- Blair, D. F., and H. C. Berg. 1990. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell 60439-449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

