Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo
- PMID: 18195357
- PMCID: PMC2242710
- DOI: 10.1073/pnas.0708862105
Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo
Abstract
Visual and auditory hallucinations accompany certain neuropsychiatric disorders, such as schizophrenia, and they also can be induced by the use or abuse of certain drugs. The heptahelical serotonin 2A receptors (5-HT2ARs) are molecular targets for drug-induced hallucinations. However, the cellular mechanisms by which the 5-HT2AR mediates these effects are not well understood. Drugs acting at the 5-HT2AR can trigger diverse signaling pathways that may be directed by the chemical properties of the drug. beta-arrestins are intracellular proteins that bind to heptahelical receptors and represent a point where such divergences in ligand-directed functional signaling could occur. Here we compare the endogenous agonist, serotonin, to a synthetic 5-HT2AR hallucinogenic agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI), in mice lacking beta-arrestin-2, as well as in cells lacking beta-arrestins. In mice, we find that serotonin induces a head twitch response by a beta-arrestin-2-dependent mechanism. However, DOI invokes the behavior independent of beta-arrestin-2. The two structurally distinct agonists elicit different signal transduction and trafficking patterns upon activation of 5-HT2AR, which hinge on the presence of beta-arrestins. Our study suggests that the 5-HT2AR-beta-arrestin interaction may be particularly important in receptor function in response to endogenous serotonin levels, which could have major implications in drug development for treating neuropsychiatric disorders such as depression and schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

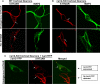
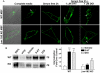
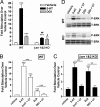
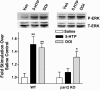
Comment in
-
Arresting serotonin.Proc Natl Acad Sci U S A. 2008 Jan 22;105(3):831-2. doi: 10.1073/pnas.0711335105. Epub 2008 Jan 14. Proc Natl Acad Sci U S A. 2008. PMID: 18195368 Free PMC article. No abstract available.
References
-
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. J Pharmacol Exp Ther. 2007;320:1–13. - PubMed
-
- Violin JD, Lefkowitz RJ. Trends Pharmacol Sci. 2007;28:416–422. - PubMed
-
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Science. 1999;286:2495–2498. - PubMed
-
- Bohn LM, Gainetdinov RR, Caron MG. Neuromol Med. 2004;5:41–50. - PubMed
-
- Raehal KM, Walker JK, Bohn LM. J Pharmacol Exp Ther. 2005;314:1195–1201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

