Rethinking early Earth phosphorus geochemistry
- PMID: 18195373
- PMCID: PMC2242691
- DOI: 10.1073/pnas.0708205105
Rethinking early Earth phosphorus geochemistry
Abstract
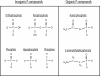
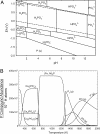
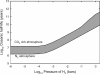
Phosphorus is a key biologic element, and a prebiotic pathway leading to its incorporation into biomolecules has been difficult to ascertain. Most potentially prebiotic phosphorylation reactions have relied on orthophosphate as the source of phosphorus. It is suggested here that the geochemistry of phosphorus on the early Earth was instead controlled by reduced oxidation state phosphorus compounds such as phosphite (HPO(3)(2-)), which are more soluble and reactive than orthophosphates. This reduced oxidation state phosphorus originated from extraterrestrial material that fell during the heavy bombardment period or was produced during impacts, and persisted in the mildly reducing atmosphere. This alternate view of early Earth phosphorus geochemistry provides an unexplored route to the formation of pertinent prebiotic phosphorus compounds, suggests a facile reaction pathway to condensed phosphates, and is consistent with the biochemical usage of reduced oxidation state phosphorus compounds in life today. Possible studies are suggested that may detect reduced oxidation state phosphorus compounds in ancient Archean rocks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Westheimer FH. Science. 1987;235:1173–1178. - PubMed
-
- Arrhenius G, Sales B, Mojzsis S, Lee T. J Theor Biol. 1997;187:503–522. - PubMed
-
- Redfield AC. Am Sci. 1958;46:205–221.
-
- Yamagata Y, Watanabe H, Saitoh M, Namba T. Nature. 1991;352:516–519. - PubMed
-
- Holland HD. Chemical Evolution of the Atmosphere and Oceans. Princeton: Princeton Univ Press; 1984.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

