Spatial alignment of the mouse blastocyst axis across the first cleavage plane is caused by mechanical constraint rather than developmental bias among blastomeres
- PMID: 18196554
- PMCID: PMC3430728
- DOI: 10.1002/mrd.20856
Spatial alignment of the mouse blastocyst axis across the first cleavage plane is caused by mechanical constraint rather than developmental bias among blastomeres
Abstract
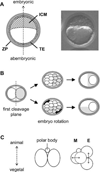
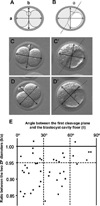
The embryonic-abembryonic (Em-Ab) axis of the mouse blastocyst has been found in several studies to align orthogonal to the first cleavage plane, raising the possibility that a developmental prepattern already exists at the two-cell stage. However, it is also possible that such alignment is not due to any developmental disparity between the two-cell stage blastomeres, but rather is caused by an extrinsic mechanical constraint that is conferred by an irregular shape of the zona pellucida (ZP). Here, we conducted a series of experiments to distinguish between these possibilities. We showed that the shape of the ZP at the two-cell stage varied among embryos, ranging from near spherical to ellipsoidal, and that the ZP shape did not change until the blastocyst stage. In those embryos with an ellipsoidal ZP, the Em-Ab axis tended to lie orthogonal to the first cleavage plane, while in those embryos with a near spherical ZP, there was no such relationship. The clonal boundary between the descendants of the two-cell stage blastomeres tended to lie orthogonal to the Em-Ab axis when the rotation of the embryo within the ZP was experimentally prevented, while the control embryos did not exhibit such tendency. These results support the possibility that an apparent correlation between the first cleavage plane and the blastocyst axis can be generated by the mechanical constraint from the ZP but not by a developmental prepattern. Moreover, recent reports indicate that the vegetal blastomere of the four-cell stage embryo that had undergone a specific type of second cleavages is destined to contribute to the abembryonic side of the blastocyst. However, our present study shows that in spite of such specific second cleavages, the vegetal blastomere did not preferentially give rise to the abembryonic side. This result implicates that the lineage of the four-cell stage blastomere is not restricted even when embryos undergo a specific type of second cleavages.
Figures


References
-
- Alarcón VB, Marikawa Y. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol Reprod. 2003;69:1208–1212. - PubMed
-
- Alarcón VB, Marikawa Y. Unbiased contribution of the first two blastomeres to mouse blastocyst development. Mol Reprod Dev. 2005;72:354–361. - PubMed
-
- Beddington RSP, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. - PubMed
-
- Chroscicka A, Komorowski S, Maleszewski M. Both blastomeres of themouse 2-cell embryo contribute to the embryonic portion of the blastocyst. Mol Reprod Dev. 2004;68:308–312. - PubMed
-
- Committee to Revise the Guide, Institute of Laboratory Animal Resources Council, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. 7th edition. Washington, DC: National Academy Press; 1996.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

