A peptide inhibitor of HIV-1 neutralizing antibody 2G12 is not a structural mimic of the natural carbohydrate epitope on gp120
- PMID: 18198210
- PMCID: PMC2430149
- DOI: 10.1096/fj.07-8983com
A peptide inhibitor of HIV-1 neutralizing antibody 2G12 is not a structural mimic of the natural carbohydrate epitope on gp120
Abstract
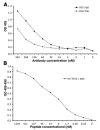
MAb 2G12 neutralizes HIV-1 by binding with high affinity to a cluster of high-mannose oligosaccharides on the envelope glycoprotein, gp120. Screening of phage-displayed peptide libraries with 2G12 identified peptides that bind specifically, with K(d)s ranging from 0.4 to 200 microM. The crystal structure of a 21-mer peptide ligand in complex with 2G12 Fab was determined at 2.8 A resolution. Comparison of this structure with previous structures of 2G12-carbohydrate complexes revealed striking differences in the mechanism of 2G12 binding to peptide vs. carbohydrate. The peptide occupies a site different from, but adjacent to, the primary carbohydrate-binding site on 2G12, and makes only slightly fewer contacts to the Fab than Man(9)GlcNAc(2) (51 vs. 56, respectively). However, only two antibody contacts with the peptide are hydrogen bonds in contrast to six with Man(9)GlcNAc(2), and only three of the antibody residues that interact with Man(9)GlcNAc(2) also contact the peptide. Thus, this mechanism of peptide binding to 2G12 does not support structural mimicry of the native carbohydrate epitope on gp120, since it neither replicates the oligosaccharide footprint on the antibody nor most of the contact residues. Moreover, 2G12.1 peptide is not an immunogenic mimic of the 2G12 epitope, since antisera produced against it did not bind gp120.
Figures
Similar articles
-
Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12.Proc Natl Acad Sci U S A. 2005 Sep 20;102(38):13372-7. doi: 10.1073/pnas.0505763102. Epub 2005 Sep 7. Proc Natl Acad Sci U S A. 2005. PMID: 16174734 Free PMC article.
-
The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120.J Virol. 2002 Jul;76(14):7306-21. doi: 10.1128/jvi.76.14.7306-7321.2002. J Virol. 2002. PMID: 12072529 Free PMC article.
-
A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN.J Virol. 2009 May;83(10):4861-70. doi: 10.1128/JVI.02537-08. Epub 2009 Mar 4. J Virol. 2009. PMID: 19264785 Free PMC article.
-
A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120.J Virol. 2008 Jul;82(13):6359-68. doi: 10.1128/JVI.00293-08. Epub 2008 Apr 23. J Virol. 2008. PMID: 18434393 Free PMC article.
-
Glycosylation of HIV-1 gp120 V3 loop: towards the rational design of a synthetic carbohydrate vaccine.Curr Med Chem. 2007;14(30):3232-42. doi: 10.2174/092986707782793826. Curr Med Chem. 2007. PMID: 18220757 Review.
Cited by
-
Potential of peptides as inhibitors and mimotopes: selection of carbohydrate-mimetic peptides from phage display libraries.J Nucleic Acids. 2012;2012:740982. doi: 10.1155/2012/740982. Epub 2012 Oct 10. J Nucleic Acids. 2012. PMID: 23094142 Free PMC article.
-
Engineering filamentous phage carriers to improve focusing of antibody responses against peptides.Vaccine. 2010 Mar 2;28(10):2174-2185. doi: 10.1016/j.vaccine.2009.12.059. Epub 2010 Jan 5. Vaccine. 2010. PMID: 20056188 Free PMC article.
-
The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design.Microbiol Mol Biol Rev. 2008 Mar;72(1):54-84, table of contents. doi: 10.1128/MMBR.00020-07. Microbiol Mol Biol Rev. 2008. PMID: 18322034 Free PMC article. Review.
-
Exploring peptide mimics for the production of antibodies against discontinuous protein epitopes.Mol Immunol. 2010 Feb;47(5):1137-48. doi: 10.1016/j.molimm.2009.10.015. Epub 2009 Dec 23. Mol Immunol. 2010. PMID: 20031219 Free PMC article.
-
Developing strategies to enhance and focus humoral immune responses using filamentous phage as a model antigen.Bioeng Bugs. 2011 Sep-Oct;2(5):275-83. doi: 10.4161/bbug.2.5.16559. Epub 2011 Sep 1. Bioeng Bugs. 2011. PMID: 22008640 Free PMC article.
References
-
- Trkola A, Pomales AB, Yuan H, Korber B, Maddon PJ, Allaway GP, Katinger H, Barbas CF, 3rd, Burton DR, Ho DD, Moore JP. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. - PMC - PubMed
-
- D'souza MP, Livnat D, Bradac JA, Bridges SH. The AIDS Clinical Trials Group Antibody Selection Working Group a. C. I. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: Performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;197:1056–1062. - PubMed
-
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. - PMC - PubMed
-
- Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. - PMC - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV 1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

