Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles
- PMID: 18198347
- PMCID: PMC2384131
- DOI: 10.1182/blood-2007-06-097410
Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles
Abstract
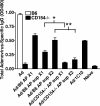
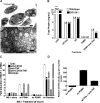
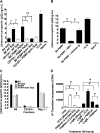
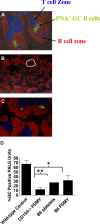
Although mounting evidence indicates that platelets participate in the modulation of both innate and adaptive immunity, the mechanisms by which platelets exert these effects have not been clearly defined. The study reported herein uses a previously documented adoptive transfer model to investigate the ability of platelet-derived membrane vesicles to communicate activation signals to the B-cell compartment. The findings demonstrate for the first time that platelet-derived membrane vesicles are sufficient to deliver CD154 to stimulate antigen-specific IgG production and modulate germinal center formation through cooperation with responses elicited by CD4(+) T cells. The data are consistent with the hypothesis that platelets modulate inflammation and adaptive immunity at sites distant from the location of activation and that platelet-derived membrane vesicles are sufficient to mediate the effect.
Figures
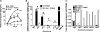
Comment in
-
Microvesicles as immune orchestra conductors.Blood. 2008 May 15;111(10):4832-3. doi: 10.1182/blood-2008-02-136028. Blood. 2008. PMID: 18467599 Free PMC article.
-
Platelet components associated with acute transfusion reactions: the role of platelet-derived soluble CD40 ligand.Blood. 2008 Dec 1;112(12):4779-80; author reply 4780-1. doi: 10.1182/blood-2008-05-157578. Blood. 2008. PMID: 19029460 No abstract available.
References
-
- George JN. Platelets. Lancet. 2000;355:1531–1539. - PubMed
-
- Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. - PubMed
-
- Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. - PubMed
-
- Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. - PubMed
-
- Elzey BD, Tian J, Jensen RJ, et al. Platelet-mediated modulation of adaptive immunity: a communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

