The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases
- PMID: 18198818
- PMCID: PMC2935787
The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases
Abstract
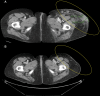
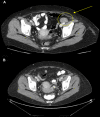
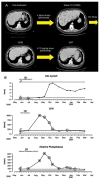
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a negative regulator of T-cell mediated immune responses and is the target of new anti-tumor immunotherapy strategies. Ipilimumab is a fully human, antagonistic monoclonal antibody directed against CTLA-4. Results from preclinical and early clinical trials support current phase II/III testing of ipilimumab as first- and second-line therapy for metastatic melanoma. Ipilimumab promotes durable objective responses and/or stable disease in patients with metastatic melanoma. Adverse events are medically manageable, largely immune-related, and presumably linked to the drug's mechanism of action. As more patients are treated with ipilimumab, it is becoming clear that the kinetics of responses are heterogeneous and significantly different from those of chemotherapy and other immunotherapy. Though objective response or stable disease is observed within 'conventional' time frames, responses have been observed weeks to months after therapy initiation. Response or stable disease may be preceded by apparent early disease progression, or may occur simultaneously with different progressing lesions within the same patient (a 'mixed' response). It is likely that the unique kinetics of response is a result of the time required to enhance and maintain an anti-tumor immune response to ipilimumab therapy. Consequently, patients may benefit from continued ipilimumab treatment through clinically known relevant disease progression or non-response during the full induction dosing schedule (12 weeks), without additional therapies. Understanding the kinetics of response to ipilimumab will help clinicians to manage patients who may benefit from treatment. In this article, several cases that illustrate the kinetics of response to ipilimumab are discussed.
References
-
- Morse MA. Technology evaluation: ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7:588–597. - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. - PubMed
-
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. - PubMed
-
- Tchekmedyian S, Glasby J, Korman A, Keler T, Deo Y, Davis T. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma [abstract].; J Clin Oncol; 2002. p. 56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials