Conformational equilibria in monomeric alpha-synuclein at the single-molecule level
- PMID: 18198943
- PMCID: PMC2174973
- DOI: 10.1371/journal.pbio.0060006
Conformational equilibria in monomeric alpha-synuclein at the single-molecule level
Abstract
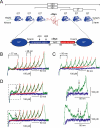
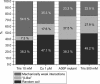
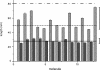
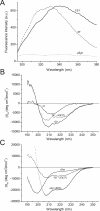
Human alpha-Synuclein (alphaSyn) is a natively unfolded protein whose aggregation into amyloid fibrils is involved in the pathology of Parkinson disease. A full comprehension of the structure and dynamics of early intermediates leading to the aggregated states is an unsolved problem of essential importance to researchers attempting to decipher the molecular mechanisms of alphaSyn aggregation and formation of fibrils. Traditional bulk techniques used so far to solve this problem point to a direct correlation between alphaSyn's unique conformational properties and its propensity to aggregate, but these techniques can only provide ensemble-averaged information for monomers and oligomers alike. They therefore cannot characterize the full complexity of the conformational equilibria that trigger the aggregation process. We applied atomic force microscopy-based single-molecule mechanical unfolding methodology to study the conformational equilibrium of human wild-type and mutant alphaSyn. The conformational heterogeneity of monomeric alphaSyn was characterized at the single-molecule level. Three main classes of conformations, including disordered and "beta-like" structures, were directly observed and quantified without any interference from oligomeric soluble forms. The relative abundance of the "beta-like" structures significantly increased in different conditions promoting the aggregation of alphaSyn: the presence of Cu2+, the pathogenic A30P mutation, and high ionic strength. This methodology can explore the full conformational space of a protein at the single-molecule level, detecting even poorly populated conformers and measuring their distribution in a variety of biologically important conditions. To the best of our knowledge, we present for the first time evidence of a conformational equilibrium that controls the population of a specific class of monomeric alphaSyn conformers, positively correlated with conditions known to promote the formation of aggregates. A new tool is thus made available to test directly the influence of mutations and pharmacological strategies on the conformational equilibrium of monomeric alphaSyn.
Conflict of interest statement
Figures
References
-
- Fink AL. Natively unfolded proteins. Curr Opin Struct Biol. 2005;15:35–41. - PubMed
-
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Cookson MR. Hero versus antihero: the multiple roles of alpha-synuclein in neurodegeneration. Exp Neurol. 2006;199:238–242. - PubMed
-
- Uversky VN, Fink AL. Protein misfolding, aggregation and conformational diseases. New York: Kluwer Academic/Plenum Publishers; 2006.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

