The use of rodent models to investigate host-bacteria interactions related to periodontal diseases
- PMID: 18199146
- PMCID: PMC2649707
- DOI: 10.1111/j.1600-051X.2007.01172.x
The use of rodent models to investigate host-bacteria interactions related to periodontal diseases
Abstract
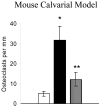
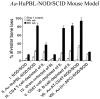
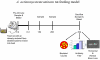
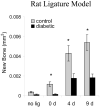
Even though animal models have limitations, they are often superior to in vitro or clinical studies in addressing mechanistic questions and serve as an essential link between hypotheses and human patients. Periodontal disease can be viewed as a process that involves four major stages: bacterial colonization, invasion, induction of a destructive host response in connective tissue and a repair process that reduces the extent of tissue breakdown. Animal studies should be evaluated in terms of their capacity to test specific hypotheses rather than their fidelity to all aspects of periodontal disease initiation and progression. Thus, each of the models described below can be adapted to test discrete components of these four major steps, but not all of them. This review describes five different animal models that are appropriate for examining components of host-bacteria interactions that can lead to breakdown of hard and soft connective tissue or conditions that limit its repair as follows: the mouse calvarial model, murine oral gavage models with or without adoptive transfer of human lymphocytes, rat ligature model and rat Aggregatibacter actinomycetemcomitans feeding model.
Figures

References
-
- Adelman N, Cohen S, Yoshida T. Strain variations in murine MIF production. J Immunol. 1978;121:209–212. - PubMed
-
- Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006;55:487–495. - PubMed
-
- Alayan J, Ivanovski S, Gemmell E, Ford P, Hamlet S, Farah CS. Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol Immunol. 2006;21:360–365. - PubMed
-
- Alikhani M, Alikhani Z, He H, Liu R, Popek BI, Graves DT. Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J Biol Chem. 2003;278:52901–52908. - PubMed
-
- Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22:775–780. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DE007559/DE/NIDCR NIH HHS/United States
- DE015254/DE/NIDCR NIH HHS/United States
- DE16191/DE/NIDCR NIH HHS/United States
- DE14473/DE/NIDCR NIH HHS/United States
- DE07559/DE/NIDCR NIH HHS/United States
- DE15566/DE/NIDCR NIH HHS/United States
- DE17732/DE/NIDCR NIH HHS/United States
- R01 DE018292/DE/NIDCR NIH HHS/United States
- R21 DE014473/DE/NIDCR NIH HHS/United States
- R01 DE015254/DE/NIDCR NIH HHS/United States
- DE016306/DE/NIDCR NIH HHS/United States
- DE018292/DE/NIDCR NIH HHS/United States
- DE15786/DE/NIDCR NIH HHS/United States
- R21 DE015786/DE/NIDCR NIH HHS/United States
- R01 DE016306/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

