A two-step process for thymic regulatory T cell development
- PMID: 18199417
- PMCID: PMC2248212
- DOI: 10.1016/j.immuni.2007.11.021
A two-step process for thymic regulatory T cell development
Abstract
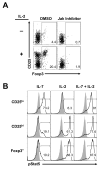
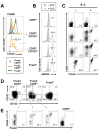
Recognition of self-antigens is required for regulatory T (Treg) cells to exert dominant tolerance. However, the mechanism by which self-reactive thymocytes are diverted into the Treg cell subset is unclear. To address this question, we looked for the immediate precursors to Treg cells within Foxp3(-)CD4+CD8(-) thymocytes. By using intrathymic transfer, we found that the CD25hi subset is highly enriched in Treg cell precursors. This was supported by tracking of thymocyte development via analysis of T cell receptor (TCR) repertoires in a TCR-beta transgenic model. These Treg cell precursors exist at a developmental stage where they are poised to express Foxp3 without further TCR engagement, requiring only stimulation by interleukin-2 (IL-2) or IL-15. Thus, we propose that the selection of self-reactive thymocytes into the Treg cell subset occurs via an instructive rather than stochastic-selective model whereby TCR signals result in the expression of proximal IL-2 signaling components facilitating cytokine-mediated induction of Foxp3.
Figures
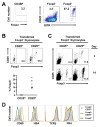

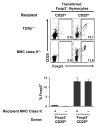
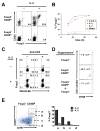
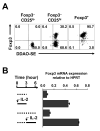
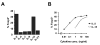
References
-
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature Immunol. 2002;3:756–763. - PubMed
-
- Bassiri H, Carding SR. A requirement for IL-2/IL-2 receptor signaling in intrathymic negative selection. J Immunol. 2001;166:5945–5954. - PubMed
-
- Bayer AL, Yu A, Malek TR. Function of the IL-2R for Thymic and Peripheral CD4+CD25+ Foxp3+ T Regulatory Cells. J Immunol. 2007;178:4062–4071. - PubMed
-
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature Genet. 2001;27:20–21. - PubMed
-
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

