Noradrenergic modulation of arousal
- PMID: 18199483
- PMCID: PMC2517224
- DOI: 10.1016/j.brainresrev.2007.10.013
Noradrenergic modulation of arousal
Abstract
Through a highly divergent efferent projection system, the locus coeruleus-noradrenergic system supplies norepinephrine throughout the central nervous system. State-dependent neuronal discharge activity of locus coeruleus neurons has long-suggested a role of this system in the induction of an alert waking state. More recent work supports this hypothesis, demonstrating robust wake-promoting actions of the locus coeruleus-noradrenergic system. Norepinephrine enhances arousal, in part, via actions of beta- and alpha1-receptors located within multiple subcortical structures, including the general regions of the medial septal area and the medial preoptic areas. Recent anatomical studies suggest that arousal-enhancing actions of norepinephrine are not limited to the locus coeruleus system and likely include the A1 and A2 noradrenergic cell groups. Thus, noradrenergic modulation of arousal state involves multiple noradrenergic systems acting within multiple subcortical regions. Pharmacological studies indicate that the combined actions of these systems are necessary for the sustained maintenance of arousal levels associated with spontaneous waking. Enhanced arousal state is a prominent aspect of both stress and psychostimulant drug action and evidence indicates that noradrenergic systems likely play an important role in both stress-related and psychostimulant-induced arousal. These and other observations suggest that the dysregulation of noradrenergic neurotransmission could well contribute to the dysregulation of arousal associated with a variety of behavioral disorders including insomnia and stress-related disorders.
Figures



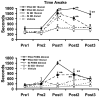
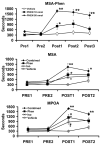

Similar articles
-
Noradrenergic modulation of wakefulness/arousal.Sleep Med Rev. 2012 Apr;16(2):187-97. doi: 10.1016/j.smrv.2011.12.003. Epub 2012 Jan 31. Sleep Med Rev. 2012. PMID: 22296742 Free PMC article. Review.
-
The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes.Brain Res Brain Res Rev. 2003 Apr;42(1):33-84. doi: 10.1016/s0165-0173(03)00143-7. Brain Res Brain Res Rev. 2003. PMID: 12668290 Review.
-
Contrasting effects of noradrenergic beta-receptor blockade within the medial septal area on forebrain electroencephalographic and behavioral activity state in anesthetized and unanesthetized rat.Neuroscience. 2000;97(3):543-52. doi: 10.1016/s0306-4522(00)00047-6. Neuroscience. 2000. PMID: 10828536
-
Organization of noradrenergic efferents to arousal-related basal forebrain structures.J Comp Neurol. 2006 Jun 10;496(5):668-83. doi: 10.1002/cne.20946. J Comp Neurol. 2006. PMID: 16615125
-
Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain.J Neurosci. 1996 Nov 1;16(21):6999-7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. J Neurosci. 1996. PMID: 8824336 Free PMC article.
Cited by
-
Bidirectional regulation of intravenous general anesthetic actions by α3-containing γ-aminobutyric acid A receptors.Anesthesiology. 2013 Mar;118(3):562-76. doi: 10.1097/ALN.0b013e3182800d76. Anesthesiology. 2013. PMID: 23303487 Free PMC article.
-
Optimization of Temporal Coding of Tactile Information in Rat Thalamus by Locus Coeruleus Activation.Biology (Basel). 2024 Jan 28;13(2):79. doi: 10.3390/biology13020079. Biology (Basel). 2024. PMID: 38392298 Free PMC article.
-
Chronic sleep restriction induces long-lasting changes in adenosine and noradrenaline receptor density in the rat brain.J Sleep Res. 2015 Oct;24(5):549-558. doi: 10.1111/jsr.12300. Epub 2015 Apr 21. J Sleep Res. 2015. PMID: 25900125 Free PMC article.
-
Autonomic Dysfunction in Sleep Disorders: From Neurobiological Basis to Potential Therapeutic Approaches.J Clin Neurol. 2022 Mar;18(2):140-151. doi: 10.3988/jcn.2022.18.2.140. J Clin Neurol. 2022. PMID: 35274834 Free PMC article. Review.
-
Phosphocreatine Levels in the Left Thalamus Decline during Wakefulness and Increase after a Nap.J Neurosci. 2018 Dec 5;38(49):10552-10565. doi: 10.1523/JNEUROSCI.0865-18.2018. Epub 2018 Oct 3. J Neurosci. 2018. PMID: 30282723 Free PMC article.
References
-
- Adams LM, Foote SL. Effects of locally infused pharmacological agents on spontaneous and sensory-evoked activity of locus coeruleus neurons. Brain Res Bull. 1988;21:395–400. - PubMed
-
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. - PubMed
-
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

