Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption
- PMID: 18199522
- PMCID: PMC2292816
- DOI: 10.1016/j.addr.2007.08.043
Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption
Abstract
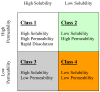
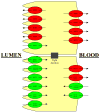
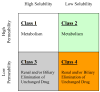
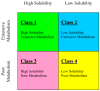
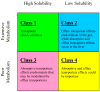
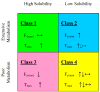
The ability to predict drug disposition involves concurrent consideration of many chemical and physiological variables and the effect of food on the rate and extent of availability adds further complexity due to postprandial changes in the gastrointestinal (GI) tract. A system that allows for the assessment of the multivariate interplay occurring following administration of an oral dose, in the presence or absence of meal, would greatly benefit the early stages of drug development. This is particularly true in an era when the majority of new molecular entities are highly permeable, poorly soluble, extensively metabolized compounds (BDDCS Class 2), which present the most complicated relationship in defining the impact of transporters due to the marked effects of transporter-enzyme interplay. This review evaluates the GI luminal environment by taking into account the absorption/transport/elimination interplay and evaluates the physiochemical property issues by taking into account the importance of solubility, permeability and metabolism. We concentrate on the BDDCS and its utility in predicting drug disposition. Furthermore, we focus on the effect of food on the extent of drug availability (F), which appears to follow closely what might be expected if a significant effect of high fat meals is inhibition of transporters. That is, high fat meals and lipidic excipients would be expected to have little effect on F for Class 1 drugs; they would increase F of Class 2 drugs, while decreasing F for Class 3 drugs.
Figures

References
-
- Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–255. - PubMed
-
- Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071–1088. - PubMed
-
- Food and Drug Administration. Guidance for Industry: Food-effect bioavailability and fed bioequivalence studies. Food and Drug Administration; Rockville, MD: 2002. Available at http://www.fda.gov/cder/guidance/index.htm.
-
- Jones HM, Parrott N, Ohlenbusch G, Lavé T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modeling. Clin Pharmacokinet. 2006;45:1213–1226. - PubMed
-
- Lentz KA, Quitko M, Morgan DG, Grace JE, Gleason C, Marathe PH. Development and validation of a preclinical food effect model. J Pharm Sci. 2007;96:459–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

