Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase
- PMID: 18199636
- PMCID: PMC2268464
- DOI: 10.1128/JVI.01753-07
Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase
Abstract
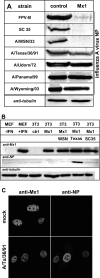
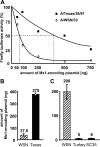
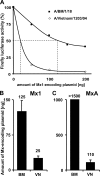
Interferon-mediated host responses are of great importance for controlling influenza A virus infections. It is well established that the interferon-induced Mx proteins possess powerful antiviral activities toward most influenza viruses. Here we analyzed a range of influenza A virus strains for their sensitivities to murine Mx1 and human MxA proteins and found remarkable differences. Virus strains of avian origin were highly sensitive to Mx1, whereas strains of human origin showed much weaker responses. Artificial reassortments of the viral components in a minireplicon system identified the viral nucleoprotein as the main target structure of Mx1. Interestingly, the recently reconstructed 1918 H1N1 "Spanish flu" virus was much less sensitive than the highly pathogenic avian H5N1 strain A/Vietnam/1203/04 when tested in a minireplicon system. Importantly, the human 1918 virus-based minireplicon system was almost insensitive to inhibition by human MxA, whereas the avian influenza A virus H5N1-derived system was well controlled by MxA. These findings suggest that Mx proteins provide a formidable hurdle that hinders influenza A viruses of avian origin from crossing the species barrier to humans. They further imply that the observed insensitivity of the 1918 virus-based replicon to the antiviral activity of human MxA is a hitherto unrecognized characteristic of the "Spanish flu" virus that may contribute to the high virulence of this unusual pandemic strain.
Figures


References
-
- Basler, C. F., A. H. Reid, J. K. Dybing, T. A. Janczewski, T. G. Fanning, H. Zheng, M. Salvatore, M. L. Perdue, D. E. Swayne, A. García-Sastre, P. Palese, and J. K. Taubenberger. 2001. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc. Natl. Acad. Sci. USA 982746-2751. - PMC - PubMed
-
- Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 46324-28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

