Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog
- PMID: 18199648
- PMCID: PMC2258991
- DOI: 10.1128/JVI.02246-07
Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog
Abstract
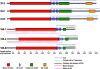
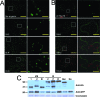
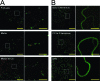
The Hsp70 homolog (Hsp70h) of Beet yellows virus (BYV) functions in virion assembly and cell-to-cell movement and is autonomously targeted to plasmodesmata in association with the actomyosin motility system (A. I. Prokhnevsky, V. V. Peremyslov, and V. V. Dolja, J. Virol. 79:14421-14428, 2005). Myosins are a diverse category of molecular motors that possess a motor domain and a tail domain involved in cargo binding. Plants have two classes of myosins, VIII and XI, whose specific functions are poorly understood. We used dominant negative inhibition to identify myosins required for Hsp70h localization to plasmodesmata. Six full-length myosin cDNAs from the BYV host plant Nicotiana benthamiana were sequenced and shown to encode apparent orthologs of the Arabidopsis thaliana myosins VIII-1, VIII-2, VIII-B, XI-2, XI-F, and XI-K. We found that the ectopic expression of the tail domains of each of the class VIII, but not the class XI, myosins inhibited the plasmodesmatal localization of Hsp70h. In contrast, the overexpression of the motor domains or the entire molecules of the class VIII myosins did not affect Hsp70h targeting. Further mapping revealed that the minimal cargo-binding part of the myosin VIII tails was both essential and sufficient for the inhibition of the proper Hsp70h localization. Interestingly, plasmodesmatal localization of the Tobacco mosaic virus movement protein and Arabidopsis protein RGP2 was not affected by myosin VIII tail overexpression. Collectively, our data implicate class VIII myosins in protein delivery to plasmodesmata and suggest that more than one mechanism of such delivery exist in plants.
Figures



References
-
- Alzhanova, D. V., Y. Hagiwara, V. V. Peremyslov, and V. V. Dolja. 2000. Genetic analysis of the cell-to-cell movement of beet yellows closterovirus. Virology 268192-200. - PubMed
-
- Avisar, D. A., I. Prokhnevsky, K. S. Makarova, E. V. Koonin, and V. V. Dolja. 4 January 2008. Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. [Epub ahead of print.] doi:10.1104/pp.107.113647. - DOI - PMC - PubMed
-
- Baulcombe, D. 2004. RNA silencing in plants. Nature 431356-363. - PubMed
-
- Bezanilla, M., A. C. Horton, H. C. Sevener, and R. S. Quatrano. 2003. Phylogenetic analysis of new plant myosin sequences. J. Mol. Evol. 57229-239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

