Targets of small interfering RNA restriction during human immunodeficiency virus type 1 replication
- PMID: 18199654
- PMCID: PMC2259002
- DOI: 10.1128/JVI.02126-07
Targets of small interfering RNA restriction during human immunodeficiency virus type 1 replication
Abstract
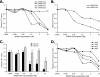
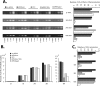
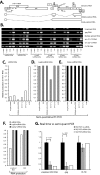
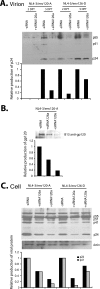
Small interfering RNAs (siRNAs) have been shown to effectively inhibit human immunodeficiency virus type 1 (HIV-1) replication in vitro. The mechanism(s) for this inhibition is poorly understood, as siRNAs may interact with multiple HIV-1 RNA species during different steps of the retroviral life cycle. To define susceptible HIV-1 RNA species, siRNAs were first designed to specifically inhibit two divergent primary HIV-1 isolates via env and gag gene targets. A self-inactivating lentiviral vector harboring these target sequences confirmed that siRNA cannot degrade incoming genomic RNA. Disruption of the incoming core structure by rhesus macaque TRIM5alpha did, however, provide siRNA-RNA-induced silencing complex access to HIV-1 genomic RNA and promoted degradation. In the absence of accelerated core disruption, only newly transcribed HIV-1 mRNA in the cytoplasm is sensitive to siRNA degradation. Inhibitors of HIV-1 mRNA nuclear export, such as leptomycin B and camptothecin, blocked siRNA restriction. All HIV-1 RNA regions and transcripts found 5' of the target sequence, including multiply spliced HIV-1 RNA, were degraded by unidirectional 3'-to-5' siRNA amplification and spreading. In contrast, HIV-1 RNA 3' of the target sequence was not susceptible to siRNA. Even in the presence of siRNA, full-length HIV-1 RNA is still encapsidated into newly assembled viruses. These findings suggest that siRNA can target only a relatively "naked" cytoplasmic HIV-1 RNA despite the involvement of viral RNA at nearly every step in the retroviral life cycle. Protection of HIV-1 RNA within the core following virus entry, during encapsidation/virus assembly, or within the nucleus may reflect virus evolution in response to siRNA, TRIM5alpha, or other host restriction factors.
Figures




Similar articles
-
Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1.Gene Ther. 2005 Jul;12(14):1133-44. doi: 10.1038/sj.gt.3302509. Gene Ther. 2005. PMID: 15750613
-
Inhibition of human immunodeficiency virus type 1 replication by siRNA targeted to the highly conserved primer binding site.Virology. 2004 Dec 5;330(1):221-32. doi: 10.1016/j.virol.2004.09.027. Virology. 2004. PMID: 15527848
-
Small interfering RNAs against the TAR RNA binding protein, TRBP, a Dicer cofactor, inhibit human immunodeficiency virus type 1 long terminal repeat expression and viral production.J Virol. 2007 May;81(10):5121-31. doi: 10.1128/JVI.01511-06. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360756 Free PMC article.
-
Current developments and future prospects for HIV gene therapy using interfering RNA-based strategies.Front Biosci. 2000 May 1;5:D527-55. doi: 10.2741/lamothe. Front Biosci. 2000. PMID: 10799355 Review.
-
Inhibition of virus replication by RNA interference.J Biomed Sci. 2003;10(6 Pt 1):607-16. doi: 10.1159/000073526. J Biomed Sci. 2003. PMID: 14576463 Review.
Cited by
-
SiRNA-induced mutation in HIV-1 polypurine tract region and its influence on viral fitness.PLoS One. 2015 Apr 10;10(4):e0122953. doi: 10.1371/journal.pone.0122953. eCollection 2015. PLoS One. 2015. PMID: 25860884 Free PMC article.
-
Enrichment of intersubtype HIV-1 recombinants in a dual infection system using HIV-1 strain-specific siRNAs.Retrovirology. 2011 Jan 13;8(1):5. doi: 10.1186/1742-4690-8-5. Retrovirology. 2011. PMID: 21232148 Free PMC article.
-
Silencing sexually transmitted infections: topical siRNA-based interventions for the prevention of HIV and HSV.Infect Dis Obstet Gynecol. 2014;2014:125087. doi: 10.1155/2014/125087. Epub 2014 Jan 12. Infect Dis Obstet Gynecol. 2014. PMID: 24526828 Free PMC article. Review.
-
Forced Complementation between Subgenomic RNAs: Does Human Immunodeficiency Type 1 Virus Reverse Transcription Occur in Viral Core, Cytoplasm, or Early Endosome?J AIDS Immune Res. 2015;1(1):101. Epub 2015 Mar 2. J AIDS Immune Res. 2015. PMID: 27239643 Free PMC article.
-
Replicative fitness and pathogenicity of primate lentiviruses in lymphoid tissue, primary human and chimpanzee cells: relation to possible jumps to humans.EBioMedicine. 2024 Feb;100:104965. doi: 10.1016/j.ebiom.2023.104965. Epub 2024 Jan 12. EBioMedicine. 2024. PMID: 38215691 Free PMC article.
References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10371-381. - PubMed
-
- Abraha, A., R. M. Troyer, M. E. Quinones-Mateu, and E. J. Arts. 2005. Methods to determine HIV-1 ex vivo fitness. Methods Mol. Biol. 304355-368. - PubMed
-
- Arts, E. J., X. Li, Z. Gu, L. Kleiman, M. A. Parniak, and M. A. Wainberg. 1994. Comparison of deoxyoligonucleotide and tRNA(Lys-3) as primers in an endogenous human immunodeficiency virus-1 in vitro reverse transcription/template-switching reaction. J. Biol. Chem. 26914672-14680. - PubMed
-
- Arts, E. J., J. Mak, L. Kleiman, and M. A. Wainberg. 1994. DNA found in human immunodeficiency virus type 1 particles may not be required for infectivity. J. Gen. Virol. 751605-1613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

