Comparing experimental and simulated pressure-area isotherms for DPPC
- PMID: 18199666
- PMCID: PMC2275714
- DOI: 10.1529/biophysj.107.114215
Comparing experimental and simulated pressure-area isotherms for DPPC
Abstract
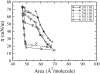
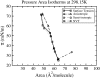
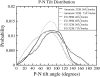
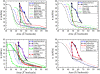
Although pressure-area isotherms are commonly measured for lipid monolayers, it is not always appreciated how much they can vary depending on experimental factors. Here, we compare experimental and simulated pressure-area isotherms for dipalmitoylphosphatidylcholine (DPPC) at temperatures ranging between 293.15 K and 323.15 K, and explore possible factors influencing the shape and position of the isotherms. Molecular dynamics simulations of DPPC monolayers using both coarse-grained (CG) and atomistic models yield results that are in rough agreement with some of the experimental isotherms, but with a steeper slope in the liquid-condensed region than seen experimentally and shifted to larger areas. The CG lipid model gives predictions that are very close to those of atomistic simulations, while greatly improving computational efficiency. There is much more variation among experimental isotherms than between isotherms obtained from CG simulations and from the most refined simulation available. Both atomistic and CG simulations yield liquid-condensed and liquid-expanded phase area compressibility moduli that are significantly larger than those typically measured experimentally, but compare well with some experimental values obtained under rapid compression.
Figures





Similar articles
-
Improved prediction of bilayer and monolayer properties using a refined BMW-MARTINI force field.Biochim Biophys Acta. 2016 Nov;1858(11):2903-2910. doi: 10.1016/j.bbamem.2016.08.016. Epub 2016 Aug 31. Biochim Biophys Acta. 2016. PMID: 27591685
-
Reparameterization of all-atom dipalmitoylphosphatidylcholine lipid parameters enables simulation of fluid bilayers at zero tension.Biophys J. 2007 Jun 15;92(12):4157-67. doi: 10.1529/biophysj.106.087130. Epub 2007 Mar 30. Biophys J. 2007. PMID: 17400696 Free PMC article.
-
A molecular dynamics study of the response of lipid bilayers and monolayers to trehalose.Biophys J. 2005 Dec;89(6):4111-21. doi: 10.1529/biophysj.105.065953. Epub 2005 Sep 23. Biophys J. 2005. PMID: 16183878 Free PMC article.
-
Molecular dynamics simulations of liquid condensed to liquid expanded transitions in DPPC monolayers.J Phys Chem B. 2010 Jan 28;114(3):1325-35. doi: 10.1021/jp9061303. J Phys Chem B. 2010. PMID: 20038155
-
Thermodynamics of the liquid states of Langmuir monolayers.J Chem Phys. 2005 Mar 8;122(10):104701. doi: 10.1063/1.1856456. J Chem Phys. 2005. PMID: 15836339
Cited by
-
Accurate Simulations of Lipid Monolayers Require a Water Model with Correct Surface Tension.J Chem Theory Comput. 2022 Mar 8;18(3):1862-1869. doi: 10.1021/acs.jctc.1c00951. Epub 2022 Feb 8. J Chem Theory Comput. 2022. PMID: 35133839 Free PMC article.
-
Adsorption of Phospholipids at the Air-Water Surface.Biophys J. 2019 Oct 1;117(7):1224-1233. doi: 10.1016/j.bpj.2019.08.022. Epub 2019 Aug 28. Biophys J. 2019. PMID: 31519299 Free PMC article.
-
Interaction forces between DPPC bilayers on glass.Langmuir. 2013 Jan 8;29(1):337-43. doi: 10.1021/la3039329. Epub 2012 Dec 14. Langmuir. 2013. PMID: 23199333 Free PMC article.
-
Water as a Link between Membrane and Colloidal Theories for Cells.Molecules. 2022 Aug 5;27(15):4994. doi: 10.3390/molecules27154994. Molecules. 2022. PMID: 35956945 Free PMC article. Review.
-
Shear-stress sensitive lenticular vesicles for targeted drug delivery.Nat Nanotechnol. 2012 Aug;7(8):536-43. doi: 10.1038/nnano.2012.84. Epub 2012 Jun 10. Nat Nanotechnol. 2012. PMID: 22683843
References
-
- Akino, T. 1992. Lipid components of the surfactant system. In Pulmonary Surfactant: From Molecular Biology to Clinical Practice. B. Robertson, L. M. G. van Golde, and J. J. Batenburg, editors. Elsevier Science Publishers, Amsterdam, The Netherlands.
-
- Keough, K. M. W. 1992. Physical chemistry of pulmonary surfactant in the terminal air spaces. In Pulmonary Surfactant: From Molecular Biology to Clinical Practice. B. Robertson, L. M. G. van Golde, and J. J. Batenburg, editors. Elsevier Science Publishers, Amsterdam, The Netherlands.
-
- Notter, R. H., S. A. Tabak, S. Holcomb, and R. D. Mavis. 1980. Postcollapse dynamic surface pressure relaxation of binary surface films containing dipalmitoyl phosphatidylcholine. J. Colloid Interface Sci. 74:370–377.
-
- Notter, R. H., R. Taubold, and R. D. Mavis. 1982. Hysteresis in saturated phospholipid films and its potential relevance for lung surfactant function in vivo. Exp. Lung Res. 3:109–127. - PubMed
-
- Knecht, V., M. Müller, M. Bonn, S. J. Marrink, and A. E. Mark. 2005. Simulation studies of pore and domain formation in a phospholipid monolayer. J. Chem. Phys. 122:24704–24712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

