Femtosecond kinetics of photoconversion of the higher plant photoreceptor phytochrome carrying native and modified chromophores
- PMID: 18199671
- PMCID: PMC2480663
- DOI: 10.1529/biophysj.106.091652
Femtosecond kinetics of photoconversion of the higher plant photoreceptor phytochrome carrying native and modified chromophores
Abstract
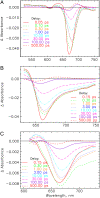
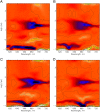
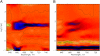
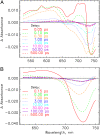
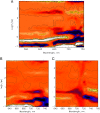
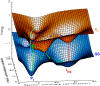
The photoprocesses of native (phyA of oat), and of C-terminally truncated recombinant phytochromes, assembled instead of the native phytochromobilin with phycocyanobilin (PCB-65 kDa-phy) and iso-phycocyanobilin (iso-PCB-65 kDa-phy) chromophores, have been studied by femtosecond transient absorption spectroscopy in both their red absorbing phytochrome (P(r)) and far-red absorbing phytochrome (P(fr)) forms. Native P(r) phytochrome shows an excitation wavelength dependence of the kinetics with three main picosecond components. The formation kinetics of the first ground-state intermediate I(700), absorbing at approximately 690 nm, is mainly described by 28 ps or 40 ps components in native and PCB phytochrome, respectively, whereas additional approximately 15 and 50 ps components describe conformational dynamics and equilibria among different local minima on the excited-state hypersurface. No significant amount of I(700) formation can be observed on our timescale for iso-PCB phytochrome. We suggest that iso-PCB-65 kDa-phy either interacts with the protein differently leading to a more twisted and/or less protonated configuration, or undergoes P(r) to P(fr) isomerization primarily via a different configurational pathway, largely circumventing I(700) as an intermediate. The isomerization process is accompanied by strong coherent oscillations due to wavepacket motion on the excited-state surface for both phytochrome forms. The femto- to (sub-)nanosecond kinetics of the P(fr) forms is again quite similar for the native and the PCB phytochromes. After an ultrafast excited-state relaxation within approximately 150 fs, the chromophores return to the first ground-state intermediate in 400-800 fs followed by two additional ground-state intermediates which are formed with 2-3 ps and approximately 400 ps lifetimes. We call the first ground-state intermediate in native phytochrome I(fr 750), due to its pronounced absorption at that wavelength. The other intermediates are termed I(fr 675) and pseudo-P(r). The absorption spectrum of the latter already closely resembles the absorption of the P(r) chromophore. PCB-65 kDa-phy shows a very similar kinetics, although many of the detailed spectral features in the transients seen in native phy are blurred, presumably due to wider inhomogeneous distribution of the chromophore conformation. Iso-PCB-65 kDa-phy shows similar features to the PCB-65 kDa-phy, with some additional blue-shift of the transient spectra of approximately 10 nm. The sub-200 fs component is, however, absent, and the picosecond lifetimes are somewhat longer than in 124 kDa phytochrome or in PCB-65 kDa-phy. We interpret the data within the framework of two- and three-dimensional potential energy surface diagrams for the photoisomerization processes and the ground-state intermediates involved in the two photoconversions.
Figures


References
-
- Batschauer, A. 1998. Photoreceptors of higher plants. Planta. 206:479–492. - PubMed
-
- Gärtner, W., and S. E. Braslavsky. 2004. The phytochromes: spectroscopy and function. In Photoreceptors and Light Signaling. A. Batschauer, editor. Royal Society of Chemistry, Cambridge, UK.
-
- Moller, S. G., P. J. Ingles, and G. C. Whitelam. 2002. The cell biology of phytochrome signaling. New Phytol. 154:553–590. - PubMed
-
- Kevei, E., and F. Nagy. 2003. Phytochrome controlled signaling cascades in higher plants. Physiol. Plant. 117:305–313. - PubMed
-
- Hughes, J., T. Lamparter, F. Mittmann, E. Hartmann, W. Gärtner, A. Wilde, and T. Börner. 1997. A prokaryotic phytochrome. Nature. 386:663. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

