Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast
- PMID: 18199682
- PMCID: PMC2291430
- DOI: 10.1091/mbc.e07-08-0805
Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast
Abstract
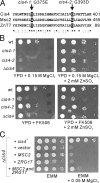
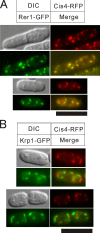
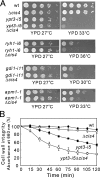
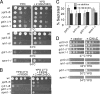
We screened for mutations that confer sensitivities to the calcineurin inhibitor FK506 and to a high concentration of MgCl(2) and isolated the cis4-1 mutant, an allele of the gene encoding a cation diffusion facilitator (CDF) protein that is structurally related to zinc transporters. Consistently, the addition of extracellular Zn(2+) suppressed the phenotypes of the cis4 mutant cells. The cis4 mutants and the mutant cells of another CDF-encoding gene SPBC16E9.14c (we named zrg17(+)) shared common and nonadditive zinc-suppressible phenotypes, and Cis4 and Zrg17 physically interacted. Cis4 localized at the cis-Golgi, suggesting that Cis4 is responsible for Zn(2+) uptake to the cis-Golgi. The cis4 mutant cells showed phenotypes such as weak cell wall and decreased acid phosphatase secretion that are thought to be resulting from impaired membrane trafficking. In addition, the cis4 deletion cells showed synthetic growth defects with all the four membrane-trafficking mutants tested, namely ypt3-i5, ryh1-i6, gdi1-i11, and apm1-1. Interestingly, the addition of extracellular Zn(2+) significantly suppressed the phenotypes of the ypt3-i5 and apm1-1 mutant cells. These results suggest that Cis4 forms a heteromeric functional complex with Zrg17 and that Cis4 is implicated in Golgi membrane trafficking through the regulation of zinc homeostasis in fission yeast.
Figures



References
-
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol. Gen. Genet. 1982;187:326–329. - PubMed
-
- Borrelly G. P., Harrison M. D., Robinson A. K., Cox S. G., Robinson N. J., Whitehall S. K. Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J. Biol. Chem. 2002;277:30394–30400. - PubMed
-
- Clemens S., Bloss T., Vess C., Neumann D., Nies D. H., Zur N. U. A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J. Biol. Chem. 2002;277:18215–18221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

