The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities
- PMID: 18199690
- PMCID: PMC2244935
- DOI: 10.1210/er.2007-0004
The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities
Erratum in
- Endocr Rev. 2009 Feb;30(1):117
Abstract
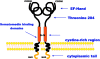
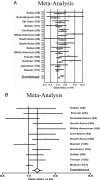
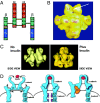
Insulin resistance is a major feature of most patients with type 2 diabetes mellitus (T2D). A number of laboratories have observed that PC-1 (membrane [corrected] glycoprotein plasma cell antigen 1; also termed [corrected] ectonucleotide pyrophosphatase phosphodiesterase 1 or ENPP1) [corrected] is either overexpressed or overactive in muscle, adipose tissue, fibroblasts, and other tissues of insulin-resistant individuals, both nondiabetic and diabetic. Moreover, PC-1 (ENPP1) overexpression [corrected] in cultured cells in vitro and in transgenic mice in vivo, [corrected] impairs insulin stimulation of insulin receptor (IR) activation and downstream signaling. PC-1 binds to the connecting domain of the IR alpha-subunit that is located in residues 485-599. The connecting domain transmits insulin binding in the alpha-subunit to activation of tyrosine kinase activation in the beta-subunit. When PC-1 is overexpressed, it inhibits insulin [corrected]induced IR beta-subunit tyrosine kinase activity. In addition, a polymorphism of PC-1 (K121Q) in various ethnic populations is closely associated with insulin resistance, T2D, and cardio [corrected] and nephrovascular diseases. The product of this polymorphism has a 2- to 3-fold increased binding affinity for the IR and is more potent than the wild-type PC-1 protein (K121K) in inhibiting the IR. These data suggest therefore that PC-1 is a candidate protein that may play a role in human insulin resistance and T2D by its overexpression, its overactivity, or both.
Figures





Similar articles
-
Mechanisms of disease: Ectonucleotide pyrophosphatase phosphodiesterase 1 as a 'gatekeeper' of insulin receptors.Nat Clin Pract Endocrinol Metab. 2006 Dec;2(12):694-701. doi: 10.1038/ncpendmet0367. Nat Clin Pract Endocrinol Metab. 2006. PMID: 17143316 Review.
-
Plasma cell membrane glycoprotein 1 (PC-1): a marker of insulin resistance in obesity, uremia and diabetes mellitus.Clin Lab. 2004;50(5-6):271-8. Clin Lab. 2004. PMID: 15209435 Review.
-
Association of ENPP1 (PC-1) K121Q polymorphism with obesity-related parameters in subjects with metabolic syndrome.Clin Endocrinol (Oxf). 2008 May;68(5):724-9. doi: 10.1111/j.1365-2265.2007.03103.x. Epub 2007 Nov 6. Clin Endocrinol (Oxf). 2008. PMID: 17986276
-
ENPP1 gene, insulin resistance and related clinical outcomes.Curr Opin Clin Nutr Metab Care. 2007 Jul;10(4):403-9. doi: 10.1097/MCO.0b013e3281e386c9. Curr Opin Clin Nutr Metab Care. 2007. PMID: 17563456 Review.
-
Evidence that inhibition of insulin receptor signaling activity by PC-1/ENPP1 is dependent on its enzyme activity.Eur J Pharmacol. 2009 Mar 15;606(1-3):17-24. doi: 10.1016/j.ejphar.2009.01.016. Epub 2009 Jan 22. Eur J Pharmacol. 2009. PMID: 19374858
Cited by
-
Variants of insulin-signaling inhibitor genes in type 2 diabetes and related metabolic abnormalities.Int J Genomics. 2013;2013:376454. doi: 10.1155/2013/376454. Epub 2013 May 23. Int J Genomics. 2013. PMID: 23762820 Free PMC article.
-
Meta-analysis of association studies between five candidate genes and type 2 diabetes in Chinese Han population.Endocrine. 2012 Oct;42(2):307-20. doi: 10.1007/s12020-012-9643-x. Epub 2012 Mar 6. Endocrine. 2012. PMID: 22391941
-
Deficiency of the bone mineralization inhibitor NPP1 protects mice against obesity and diabetes.Dis Model Mech. 2014 Dec;7(12):1341-50. doi: 10.1242/dmm.017905. Epub 2014 Oct 31. Dis Model Mech. 2014. PMID: 25368121 Free PMC article.
-
Transcriptomic and epigenomic signatures of liver metabolism and insulin sensitivity in aging mice.Mech Ageing Dev. 2025 Jun;225:112068. doi: 10.1016/j.mad.2025.112068. Epub 2025 May 3. Mech Ageing Dev. 2025. PMID: 40324540
-
Synthesis of new class of indole acetic acid sulfonate derivatives as ectonucleotidases inhibitors.RSC Adv. 2023 Oct 10;13(42):29496-29511. doi: 10.1039/d3ra04266a. eCollection 2023 Oct 4. RSC Adv. 2023. PMID: 37822663 Free PMC article.
References
-
- Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM 2000 Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care 23:171–175 - PubMed
-
- Bogardus C, Lillioja S, Mott DM, Hollenbeck C, Reaven G 1985 Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol 248:E286–E291 - PubMed
-
- Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Knowler WC, Bennett PH, Moll P, Bogardus C 1987 In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes 36:1329–1335 - PubMed
-
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR 1990 Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113:909–915 - PubMed
-
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C 1993 Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329:1988–1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

