A proton-mediated conformational shift identifies a mobile pore-lining cysteine residue (Cys-561) in human concentrative nucleoside transporter 3
- PMID: 18199742
- PMCID: PMC2417190
- DOI: 10.1074/jbc.M710433200
A proton-mediated conformational shift identifies a mobile pore-lining cysteine residue (Cys-561) in human concentrative nucleoside transporter 3
Abstract
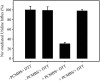
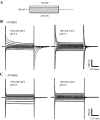
The concentrative nucleoside transporter (CNT) protein family in humans is represented by three members, hCNT1, hCNT2, and hCNT3. Belonging to a CNT subfamily phylogenetically distinct from hCNT1/2, hCNT3 mediates transport of a broad range of purine and pyrimidine nucleosides and nucleoside drugs, whereas hCNT1 and hCNT2 are pyrimidine and purine nucleoside-selective, respectively. All three hCNTs are Na(+)-coupled. Unlike hCNT1/2, however, hCNT3 is also capable of H(+)-mediated nucleoside cotransport. Using site-directed mutagenesis in combination with heterologous expression in Xenopus oocytes, we have identified a C-terminal intramembranous cysteine residue of hCNT3 (Cys-561) that reversibly binds the hydrophilic thiol-reactive reagent p-chloromercuribenzene sulfonate (PCMBS). Access of this membrane-impermeant probe to Cys-561, as determined by inhibition of hCNT3 transport activity, required H(+), but not Na(+), and was blocked by extracellular uridine. Although this cysteine residue is also present in hCNT1 and hCNT2, neither transporter was affected by PCMBS. We conclude that Cys-561 is located in the translocation pore in a mobile region within or closely adjacent to the nucleoside binding pocket and that access of PCMBS to this residue reports a specific H(+)-induced conformational state of the protein.
Figures









Similar articles
-
Substituted cysteine accessibility method analysis of human concentrative nucleoside transporter hCNT3 reveals a novel discontinuous region of functional importance within the CNT family motif (G/A)XKX3NEFVA(Y/M/F).J Biol Chem. 2009 Jun 19;284(25):17281-17292. doi: 10.1074/jbc.M109.009704. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380585 Free PMC article.
-
A conformationally mobile cysteine residue (Cys-561) modulates Na+ and H+ activation of human CNT3.J Biol Chem. 2008 Sep 5;283(36):24922-34. doi: 10.1074/jbc.M801793200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621735 Free PMC article.
-
Substituted cysteine accessibility method (SCAM) analysis of the transport domain of human concentrative nucleoside transporter 3 (hCNT3) and other family members reveals features of structural and functional importance.J Biol Chem. 2017 Jun 9;292(23):9505-9522. doi: 10.1074/jbc.M116.743997. Epub 2017 Apr 6. J Biol Chem. 2017. PMID: 28385889 Free PMC article.
-
Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib).Mol Membr Biol. 2001 Jan-Mar;18(1):65-72. doi: 10.1080/09687680010026313. Mol Membr Biol. 2001. PMID: 11396613 Review.
-
SLC28 genes and concentrative nucleoside transporter (CNT) proteins.Xenobiotica. 2008 Jul;38(7-8):972-94. doi: 10.1080/00498250802069096. Xenobiotica. 2008. PMID: 18668436 Review.
Cited by
-
Conserved glutamate residues Glu-343 and Glu-519 provide mechanistic insights into cation/nucleoside cotransport by human concentrative nucleoside transporter hCNT3.J Biol Chem. 2009 Jun 19;284(25):17266-17280. doi: 10.1074/jbc.M109.009613. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380587 Free PMC article.
-
Red fluorescent protein pH biosensor to detect concentrative nucleoside transport.J Biol Chem. 2009 Jul 31;284(31):20499-511. doi: 10.1074/jbc.M109.019042. Epub 2009 Jun 3. J Biol Chem. 2009. PMID: 19494110 Free PMC article.
-
A metal-containing nucleoside that possesses both therapeutic and diagnostic activity against cancer.J Biol Chem. 2015 Apr 10;290(15):9714-26. doi: 10.1074/jbc.M114.620294. Epub 2015 Feb 24. J Biol Chem. 2015. PMID: 25713072 Free PMC article.
-
Substituted cysteine accessibility method analysis of human concentrative nucleoside transporter hCNT3 reveals a novel discontinuous region of functional importance within the CNT family motif (G/A)XKX3NEFVA(Y/M/F).J Biol Chem. 2009 Jun 19;284(25):17281-17292. doi: 10.1074/jbc.M109.009704. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380585 Free PMC article.
-
A conformationally mobile cysteine residue (Cys-561) modulates Na+ and H+ activation of human CNT3.J Biol Chem. 2008 Sep 5;283(36):24922-34. doi: 10.1074/jbc.M801793200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621735 Free PMC article.
References
-
- Cass, C. E. (1995) in Drug Transport in Antimicrobial and Anticancer Chemotherapy (Georgopapadakou, N. H., ed) pp. 403-451, Marcel Dekker, Inc., New York
-
- Griffith, D. A., and Jarvis, S. M. (1996) Biochim. Biophys. Acta 1286 153-181 - PubMed
-
- Young, J. D., Cheeseman, C. I., Mackey, J. R., Cass, C. E., and Baldwin, S. A. (2000) in Current Topics in Membranes (Barrett, K. E., and Donowitz, M., eds) Vol. 50, pp. 329-378, Academic Press, Inc., San Diego
-
- Damaraju, V. L., Damaraju, S., Young, J. D., Baldwin, S. A., Mackey, J., Sawyer, M. B., and Cass, C. E. (2003) Oncogene 22 7524-7536 - PubMed
-
- Latini, S., and Pedata, F. (2001) J. Neurochem. 79 463-484 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

