tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis
- PMID: 18199803
- PMCID: PMC2391054
- DOI: 10.1681/ASN.2007030300
tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis
Abstract
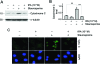
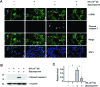
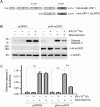
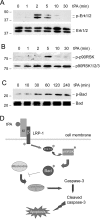
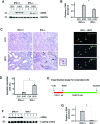
Activation and expansion of interstitial fibroblasts and myofibroblasts play an essential role in the evolution of renal fibrosis. After obstructive injury, mice lacking tissue-type plasminogen activator (tPA) have fewer myofibroblasts and less interstitial fibrosis than wild-type controls. This suggests that tPA controls the size of the fibroblast/myofibroblast population in vivo, and this study sought to determine the underlying mechanism. In vitro, tPA inhibited staurosporine or H(2)O(2)-induced caspase-3 activation, prevented cellular DNA fragmentation, and suppressed the release of cytochrome C from mitochondria into the cytosol in a rat interstitial fibroblast cell line (NRK-49F). tPA also protected TGF-beta1-activated myofibroblasts from apoptosis. This antiapoptotic effect of tPA was independent of its protease activity but required its membrane receptor, the LDL receptor-related protein 1 (LRP-1). Deletion or knockdown of LRP-1 abolished tPA-mediated cell survival, whereas re-introduction of an LRP-1 minigene in a mouse LRP-1-deficient fibroblast cell line (PEA-13) restored the cytoprotective ability of tPA. tPA triggered a cascade of survival signaling involving extracellular signal-regulated kinase 1/2 (Erk1/2), p90RSK, and phosphorylation of Bad. Blockade of Erk1/2 activation abrogated the antiapoptotic effect of tPA, whereas expression of constitutively active MEK1 promoted cell survival similar to tPA. In vivo, compared with wild-type controls, apoptosis of interstitial myofibroblasts was increased in tPA(-/-) mice after obstructive injury, and myofibroblasts were completely depleted 4 wk after relief of the obstruction. Together, these findings illustrate that tPA is a survival factor that prevents apoptosis of renal interstitial fibroblasts and myofibroblasts through an LRP-1-, Erk1/2-, p90RSK-, and Bad-dependent mechanism.
Figures

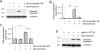


References
-
- Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 - PubMed
-
- Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 - PubMed
-
- Strutz F, Zeisberg M: Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006 - PubMed
-
- Qi W, Chen X, Poronnik P, Pollock CA: The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 38: 1–5, 2006 - PubMed
-
- Neilson EG: Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

