The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea
- PMID: 18199833
- PMCID: PMC2242701
- DOI: 10.1073/pnas.0709813105
The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea
Abstract
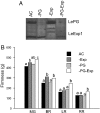
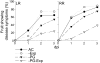
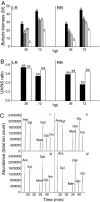
Fruit ripening is characterized by processes that modify texture and flavor but also by a dramatic increase in susceptibility to necrotrophic pathogens, such as Botrytis cinerea. Disassembly of the major structural polysaccharides of the cell wall (CW) is a significant process associated with ripening and contributes to fruit softening. In tomato, polygalacturonase (PG) and expansin (Exp) are among the CW proteins that cooperatively participate in ripening-associated CW disassembly. To determine whether endogenous CW disassembly influences the ripening-regulated increase in necrotropic pathogen susceptibility, B. cinerea susceptibility was assessed in transgenic fruit with suppressed polygalacturonase (LePG) and expansin (LeExp1) expression. Suppression of either LePG or LeExp1 alone did not reduce susceptibility but simultaneous suppression of both dramatically reduced the susceptibility of ripening fruit to B. cinerea, as measured by fungal biomass accumulation and by macerating lesion development. These results demonstrate that altering endogenous plant CW disassembly during ripening influences the course of infection by B. cinerea, perhaps by changing the structure or the accessibility of CW substrates to pathogen CW-degrading enzymes. Recognition of the role of ripening-associated CW metabolism in postharvest pathogen susceptibility may be useful in the design and development of strategies to limit pathogen losses during fruit storage, handling, and distribution.
Conflict of interest statement
The authors declare no conflict of interest.
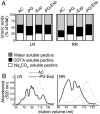
Figures

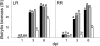

References
-
- Vorwerk S, Somerville S, Somerville C. Trends Plant Sci. 2004;9:203–209. - PubMed
-
- Hückelhoven R, editor. Annu Rev Phytopath. 2007;45:101–127. - PubMed
-
- Collmer A, Keen NT. Annu Rev Phytopath. 1986;24:383–409.
-
- Brummell DA, Howie WJ, Ma C, Dunsmuir P. Postharvest Biol Tech. 2002;25:209–220.
-
- ten Have A, Mulder W, Visser J, van Kan JAL. Mol Plant–Microbe Interact. 1998;11:1009–1016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

