S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response
- PMID: 18199853
- PMCID: PMC6361142
- DOI: 10.1124/mi.7.6.7
S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response
Abstract
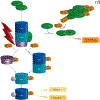
The specific posttranslational modification of protein cysteine residues by the addition of the tripeptide glutathione is termed S-glutathionylation. This process is promoted by oxidative and nitrosative stress but also occurs in unstressed cells. Altered levels of S-glutathionylation in some proteins have been associated with numerous pathologies, many of which have been linked to redox stress in the endoplasmic reticulum (ER). Proper protein folding is dependent upon controlled redox conditions within the ER, and it seems that ER conditions can in turn affect rates of S-glutathionylation. This article seeks to bring together the ways through which these processes are interrelated and considers the implications of these interrelationships upon therapeutic approaches to disease.
Figures
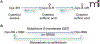


References
-
- Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, and Murphy MP Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem 279, 47939–47951 (2004). - PubMed
-
- Shelton MD, Chock PB, and Mieyal JJ Glutaredoxin: Role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox. Signal 7, 348–366 (2005). - PubMed
-
- Tew KD Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 54, 4313–4320 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
