Regulation of spike timing in visual cortical circuits
- PMID: 18200026
- PMCID: PMC2868969
- DOI: 10.1038/nrn2315
Regulation of spike timing in visual cortical circuits
Abstract
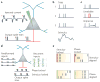
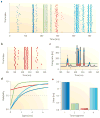
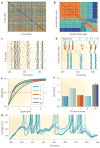
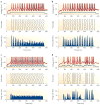
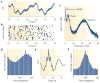
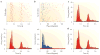
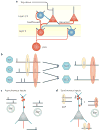
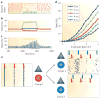
A train of action potentials (a spike train) can carry information in both the average firing rate and the pattern of spikes in the train. But can such a spike-pattern code be supported by cortical circuits? Neurons in vitro produce a spike pattern in response to the injection of a fluctuating current. However, cortical neurons in vivo are modulated by local oscillatory neuronal activity and by top-down inputs. In a cortical circuit, precise spike patterns thus reflect the interaction between internally generated activity and sensory information encoded by input spike trains. We review the evidence for precise and reliable spike timing in the cortex and discuss its computational role.
Figures
References
-
- Mainen Z, Sejnowski T. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. - PubMed
-
- Liu RC, Tzonev S, Rebrik S, Miller KD. Variability and information in a neural code of the cat lateral geniculate nucleus. J Neurophysiol. 2001;86:2789–2806. - PubMed
-
- Butts DA, et al. Temporal precision in the neural code and the timescales of natural vision. Nature. 2007;449:92–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

