Peptide-assisted degradation of the Salmonella MgtC virulence factor
- PMID: 18200043
- PMCID: PMC2241655
- DOI: 10.1038/sj.emboj.7601983
Peptide-assisted degradation of the Salmonella MgtC virulence factor
Abstract
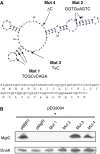
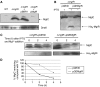
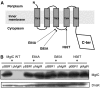
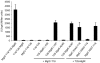
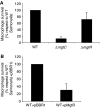
MgtC is a virulence factor common to several intracellular pathogens that is required for intramacrophage survival and growth in magnesium-depleted medium. In Salmonella enterica, MgtC is coexpressed with the MgtB magnesium transporter and transcription of the mgtCB operon is induced by magnesium deprivation. Despite the high level of mgtCB transcriptional induction in magnesium-depleted medium, the MgtC protein is hardly detected in a wild-type Salmonella strain. Here, we show that downregulation of MgtC expression is dependent on a hydrophobic peptide, MgtR, which is encoded by the mgtCB operon. Our results suggest that MgtR promotes MgtC degradation by the FtsH protease, providing a negative regulatory feedback. Bacterial two-hybrid assays demonstrate that MgtR interacts with the inner-membrane MgtC protein. We identified mutant derivatives of MgtR and MgtC that prevent both regulation and interaction between the two partners. In macrophages, overexpression of the MgtR peptide led to a decrease of the replication rate of Salmonella. This study highlights the role of peptides in bacterial regulatory mechanisms and provides a natural antagonist of the MgtC virulence factor.
Figures




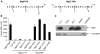
References
-
- Adkins JN, Mottaz HM, Norbeck AD, Gustin JK, Rue J, Clauss TR, Purvine SO, Rodland KD, Heffron F, Smith RD (2006) Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol Cell Proteomic 5: 1450–1461 - PubMed
-
- Aldridge P, Karlinsey J, Hughes KT (2003) The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol Microbiol 49: 1333–1345 - PubMed
-
- Alix E, Blanc-Potard AB (2007) MgtC: a key player in intramacrophage survival. Trends Microbiol 15: 252–256 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

