Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis
- PMID: 18200044
- PMCID: PMC2241654
- DOI: 10.1038/sj.emboj.7601982
Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis
Abstract
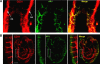
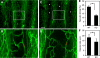
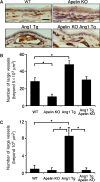
Blood vessels change their caliber to adapt to the demands of tissues or organs for oxygen and nutrients. This event is mainly organized at the capillary level and requires a size-sensing mechanism. However, the molecular regulatory mechanism involved in caliber size modification in blood vessels is not clear. Here we show that apelin, a protein secreted from endothelial cells under the activation of Tie2 receptor tyrosine kinase on endothelial cells, plays a role in the regulation of caliber size of blood vessel through its cognate receptor APJ, which is expressed on endothelial cells. During early embryogenesis, APJ is expressed on endothelial cells of the new blood vessels sprouted from the dorsal aorta, but not on pre-existing endothelial cells of the dorsal aorta. Apelin-deficient mice showed narrow blood vessels in intersomitic vessels during embryogenesis. Apelin enhanced endothelial cell proliferation in the presence of vascular endothelial growth factor and promoted cell-to-cell aggregation. These results indicated that the apelin/APJ system is involved in the regulation of blood vessel diameter during angiogenesis.
Figures



Similar articles
-
Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes.FASEB J. 2008 Dec;22(12):4146-53. doi: 10.1096/fj.07-104018. Epub 2008 Aug 15. FASEB J. 2008. PMID: 18708591
-
Maturation of blood vessels by haematopoietic stem cells and progenitor cells: involvement of apelin/APJ and angiopoietin/Tie2 interactions in vessel caliber size regulation.Thromb Haemost. 2009 Jun;101(6):999-1005. Thromb Haemost. 2009. PMID: 19492139 Review.
-
Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia.Blood. 2010 Apr 15;115(15):3166-74. doi: 10.1182/blood-2009-07-232306. Epub 2010 Feb 25. Blood. 2010. PMID: 20185589
-
Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis.Circ Res. 2008 Aug 15;103(4):432-40. doi: 10.1161/CIRCRESAHA.108.179333. Epub 2008 Jul 10. Circ Res. 2008. PMID: 18617693
-
[Apelin signalisation and vascular physiopathology].J Soc Biol. 2009;203(2):171-9. doi: 10.1051/jbio/2009021. Epub 2009 Jun 16. J Soc Biol. 2009. PMID: 19527631 Review. French.
Cited by
-
Vascular anomalies: from genetics toward models for therapeutic trials.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a009688. doi: 10.1101/cshperspect.a009688. Cold Spring Harb Perspect Med. 2012. PMID: 22908197 Free PMC article. Review.
-
BMP9 and BMP10 are critical for postnatal retinal vascular remodeling.Blood. 2012 Jun 21;119(25):6162-71. doi: 10.1182/blood-2012-01-407593. Epub 2012 May 7. Blood. 2012. PMID: 22566602 Free PMC article.
-
Glomerular endothelial cell maturation depends on ADAM10, a key regulator of Notch signaling.Angiogenesis. 2018 May;21(2):335-347. doi: 10.1007/s10456-018-9599-4. Epub 2018 Feb 3. Angiogenesis. 2018. PMID: 29397483 Free PMC article.
-
The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary.Int J Biol Sci. 2009 May 13;5(4):344-50. doi: 10.7150/ijbs.5.344. Int J Biol Sci. 2009. PMID: 19461937 Free PMC article.
-
Cancer apelin receptor suppresses vascular mimicry in malignant melanoma.Pathol Oncol Res. 2023 Jan 27;29:1610867. doi: 10.3389/pore.2023.1610867. eCollection 2023. Pathol Oncol Res. 2023. PMID: 36776217 Free PMC article.
References
-
- Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R, Greve J, Robbins R, Patterson AJ, Bernstein D, Quertermous T (2005) The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 65: 73–82 - PMC - PubMed
-
- Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9: 653–660 - PubMed
-
- Cho CH, Kim KE, Byun J, Jang HS, Kim DK, Baluk P, Baffert F, Lee GM, Mochizuki N, Kim J, Jeon BH, McDonald DM, Koh GY (2005) Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res 97: 86–94 - PubMed
-
- Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA (2006) Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol 296: 177–189 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

